Neurilemmoma of the Vagus Nerve in the Poststyloid Parapharyngeal Space
Yuji Shinohara1, Takashi Matsumoto2, Norifumi Kiga3, Itaru Tojyo4, Shigeyuki Fujita5
1 Research Associate, Department of Oral and Maxillofacial Surgery, Wakayama Medical University, Wakayama, Wakayama Prefecture, Japan.
2 Research Associate, Department of Oral and Maxillofacial Surgery, Wakayama Medical University, Wakayama, Wakayama Prefecture, Japan.
3 Assistant Professor, Department of Oral and Maxillofacial Surgery, Wakayama Medical University, Wakayama, Wakayama Prefecture, Japan.
4 Assistant Professor, Department of Oral and Maxillofacial Surgery, Wakayama Medical University, Wakayama, Wakayama Prefecture, Japan.
5 Professor, Department of Oral and Maxillofacial Surgery, Wakayama Medical University, Wakayama, Wakayama Prefecture, Japan.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Yuji Shinohara, 811-1, Kimiidera, Wakayama-City 641-8509, Japan. E-mai : ijuyar@yahoo.co.jp
We report a large vagal neurilemmoma in the poststyloid compartment of the parapharyngeal space. A 52-year-old man was referred to our hospital with a feeling of discomfort in the left upper neck. Computed tomography showed a 30mm x 30mm x 40mm mass with inhomogeneous internal enhancement in the left carotid space. Magnetic resonance imaging revealed a 30mm × 30mm × 40mm heterogeneous mass in the area of the bifurcation of the common carotid artery. We gave a provisional diagnosis of neurilemmoma or vagal paraganglioma in the parapharyngeal space preoperatively based on the results of physical examination and imaging. We selected a transcervical-transmandibular approach. Under general anaesthesia, a tumour originating from the vagus nerve was completely extirpated while protecting the internal and external carotid arteries. Although mild postvagotomy dysphagia and hoarseness were seem for 6 months postoperatively, symptoms resolved and the patient showed a satisfactory course without recurrence after 10 years. Histological examination of the excised specimen showed antoni A and antoni B pattern. Positive immunoreactivity for S-100 protein was identified, but negative results were obtained for neuron-specific enolase, chromogranin and neurofilament. The tumour was diagnosed as neurilemmoma of the vagus nerve.
Neurilemmoma, Paraganglioma, Poststyloid parapharyngeal space, Pre-mental foramen mandibulotomy
Case Report
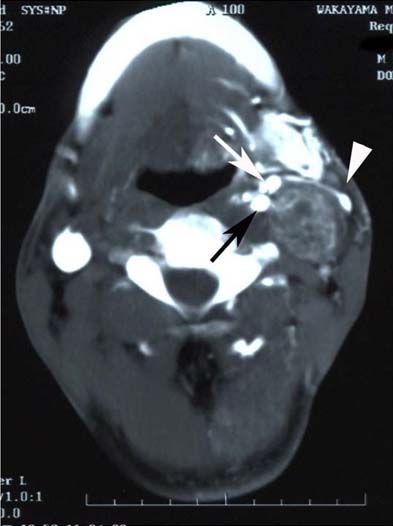
A 52-year-old man was referred to the Department of Oral and Maxillofacial Surgery at Wakayama Medical University with a chief complaint of discomfort in left side of the neck. The patient did not have any previous history of illness. A movable mass in the left upper neck, in the region of the carotid bifurcation and anterior border of the sternocleidomastoid muscle, was detected on palpation, but the patient reported no other symptoms. Computed Tomography (CT) showed a 30mm 30mm 40mm mass with inhomogeneous internal enhancement in the left carotid space. The internal and external carotid arteries were displaced anteriorly, and the internal jugular vein was displaced laterally by the mass [Table/Fig-1]. On Magnetic Resonance Imaging (MRI), the mass was almost the same size as on CT. The mass exhibited scattered signal hypointensity within signal hyperintensity on T1-weighted MRI [Table/Fig-2a]. On T2-weighted MRI, the mass exhibited scattered areas of hyperintensity and hypointensity suggestive of capillary vessels [Table/Fig-2b]. In other words, the view coronal-section T1-weighted MRI shows the tumour in the parapharyngeal space inside the mandible [Table/Fig-2c].
Contrast-enhanced CT of the neck showing a left mass (30mm x 30mm x 40mm) displacing laterally the internal jugular vein (arrowhead) and displacing anteriorly the internal carotid artery (black arrow) and external carotid artery (white arrow).
a) Axial T1-weighted MRI shows internally scattered signal hypointensity. b) Axial T2-weighted MRI shows internally scattered signal hyper and hypointensities suggestive of capillary vessels (black arrows). c) Coronal-section T1-weighted MRI shows the tumour in the parapharyngeal space (black arrows) inside the mandible (white arrows). d) Sagittal-section T1-weighted MRI shows the tumour adjacent to the skull base.
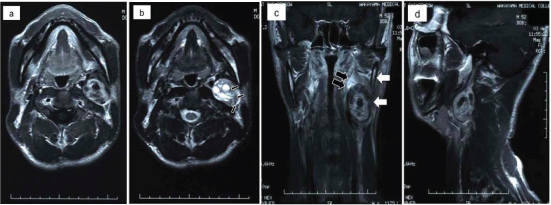
MRI and CT findings for the tumour closely resembled those characteristics of paraganglioma. We consulted radiologists regarding the possibility of paraganglioma and neurosurgeons to clarify positional relationships of the skull base and internal and external carotid arteries. The advice from radiologists and neurosurgeons was that the tumour was operable because of the lack of adhesions to major vessels. The tumour had not infiltrated the skull base [Table/Fig-2d]. Using a load test of internal carotid occlusion, we confirmed that ligation of the internal carotid artery would not result in brain damage. Informed consent was obtained before surgery after providing these results. We gave a provisional diagnosis of neurilemmoma or vagal paraganglioma in the poststyloid parapharyngeal space preoperatively based on the results of physical examination and imaging.
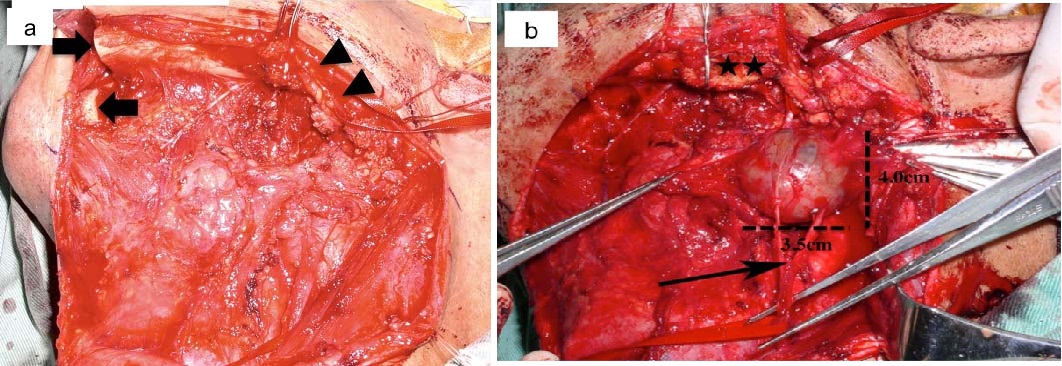
Surgical excision of the tumour was performed under general anaesthesia. For the surgical approach to the tumour, we selected a transcervical-transmandibular approach. We needed an excision of tissue in the parapharyngeal space, if the lesion turned out to be malignant tumour. The tumour was a membrane-covered lesion and relatively easy to separate from the internal jugular vein. However, we severed the vagus nerve running into the tumour, because separation from the tumour proved difficult [Table/Fig-3a,b].
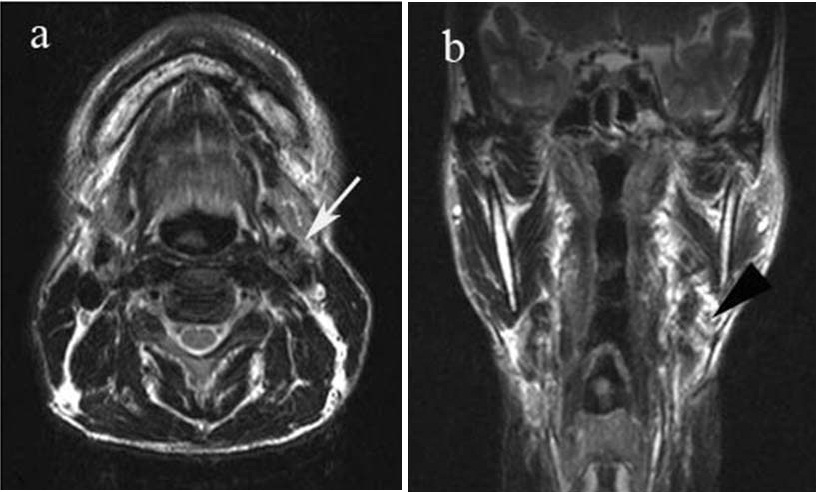
No other difficulties were encountered intraoperatively, and no mandibular nerve paralysis was evident postoperatively. Although mild postvagotomy dysphagia and hoarseness were seem for 6 months postoperatively, symptoms resolved and the patient showed a satisfactory course without recurrence after 10 years [Table/Fig-4a,b].
a) Transcervical-transmandibular approach. Black arrows indicate the mandibular stump. Arrowheads show marginal mandibular branches of the facial nerve. The mandible on the affected side is easily pushed up posterosuperiorly. b) Operative view shows tumour involving the vagus nerve (arrow). Using a transcervical-transmandibular approach, the mandible was pulled posterosuperiorly with a single hook (stars). This allowed acquisition of a wide, clear surgical field of the parapharyngeal space inside the mandible.
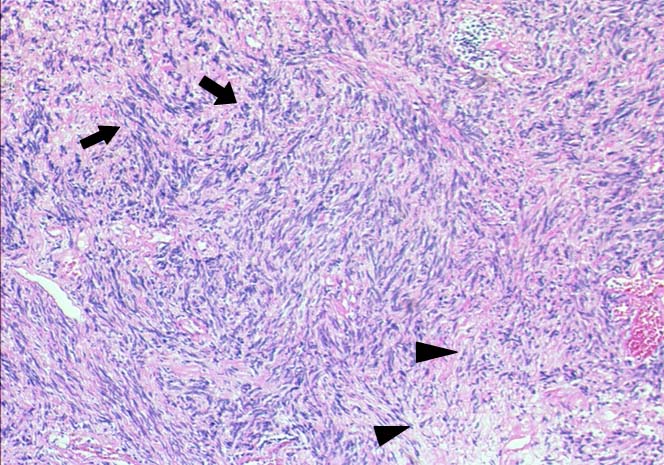
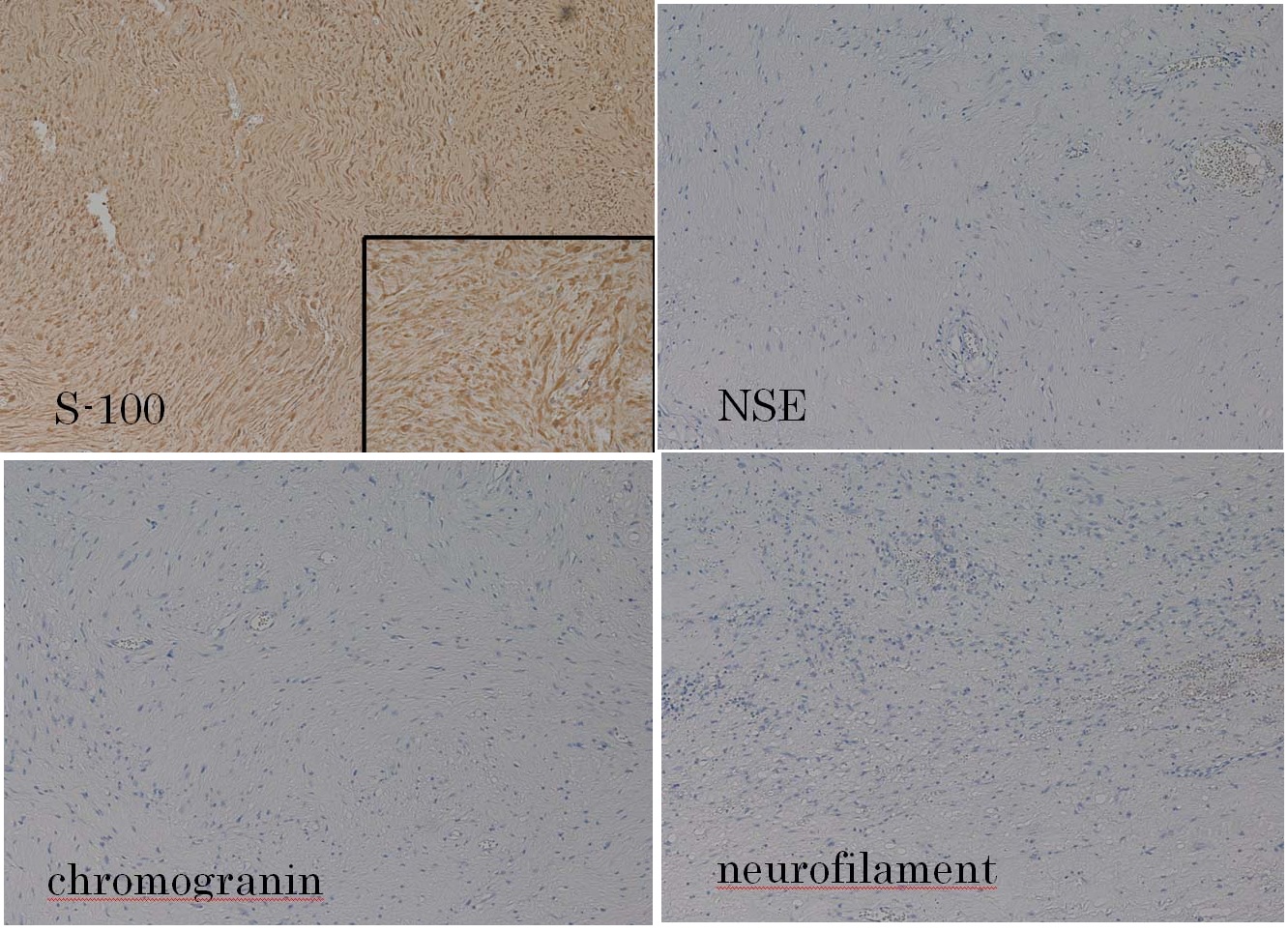
Histological examination of the excised specimen showed antoni A and antoni B pattern. [Table/Fig-5]. Furthermore, we performed the immunostaining of the specimen. A positive reaction to S-100 protein was confirmed on immunostaining, but negative results were obtained for Neuron-Specific Enolase (NSE), chromogranin and neurofilament [Table/Fig-6]. These histological and immunohistochemical findings excluded the diagnosis of vagal paraganglioma. The tumour was given a final diagnosis as neurilemmoma of the vagus nerve.
a) Axial T1-weighted MRI shows the recurrence of tumour was not seen 1 year after operation (white arrows). b) Coronal-section T1-weighted MRI shows the tumour in the parapharyngeal space disappeared. (black arrowhead)
Histopathological examination of the excised specimen shows antoni A and antoni B pattern of the neurilemmomas. Black arrows indicate antoni A pattern, and arrowheads show antoni B pattern (hematoxylin and eosin stain, original magnification, x100)
Immunohistochemical examination of the excised specimen shows positive reactions to S-100 protein and negative reactions to NSE, chromogranin and neurofilament (original magnification, x100). Positive staining for S-100 protein indicating neural origin of the tumour (insert)
Discussion
Neurilemmoma is a slowly growing, encapsulated tumour arising from the perineural cells located in the periphereal nerve sheath. Neurilemmoma of the vaugs nerve is a relatively rare lesion. Whitaker and Droulias reported 76 neurilemmomas, of which only 4 arouse from the cervical vargus nerve [1]. Katz et al., reported 15 extracranial neurogenous neoplasms, of which 5 were neurilemmoma originating from the vagus nerve [2]. Vagal paraganglioma is a neuroendocrine neoplasm derived from the paraganglia found within or adjacent to the vagus nerve usually in the vicinity of the ganglion nodosum. Vagal paraganglioma are highly vascular and are located in the suprahyoid neck, well above the level of the carotid bifurcation and typically displace both external and internal carotid arteries anteromedially [3]. The accurate preoperative diagnosis is difficult in a lesion developing in the parapharyngeal space, because both of neurilemmoma and vagal paraganglioma are vagus nerve origin.
On CT, the neurilemmoma lesion typically presents as a large, sharply demarcated mass, round or oval, showing isoattenuation with muscle, sometimes cystic, and often heterogeneously enhancing [4]. On MRI, neurilemmomas are well-circumscribed homogenous masses that exhibit signal hyperintensity on T2-weighted imaging and relatively homogeneous signal hypointensity on T1-weighted imaging. No vascular flow voids are seen in neurilemmoma in contrast with paragangliomas [5]. In our case, the capillary vessel regions in the mass were apparent on radiologic examination. Differentiating neurilemmoma from paraganglioma was therefore difficult.
The parapharyngeal space extends from the skull base to the hyoid bone and is bound medially by the fascia surrounding the pharyngeal constrictors and laterally by the ramus of the mandible and the medial pterygoid muscle. The parapharyngeal space has two compartments, a prestyloid compartment and poststyloid compartment, separated by the styloid process [6]. In cases of a large tumour in the poststyloid compartment of the parapharyngeal space, surgical procedures are difficult due to proximity to the skull base and the high risk of damage to the internal and external carotid arteries, internal jugular vein and lower cranial nerves. Haemorrhage from major blood vessels may have occurred under an unclear surgical field, because the large tumour was surrounded by the internal and external carotid arteries and internal jugular vein. In addition, the transcervical approach and mandibular resection are reportedly useful as surgical procedures for malignant tumours in the parapharyngeal space [7]. In this case, we selected a transcervical-transmandibular approach for the following reasons: the ability to obtain a wide surgical field, the ease of pushing up the mandible on the affected side with little damage to surrounding soft tissue, and smaller cosmetic disturbance. Actually, the wide and clear surgical field of the parapharyngeal space inside the mandible was essential in this operation [Table/Fig-3a,b].
In this case, histological examination of the specimens showed antoni A and antoni B pattern. Furthermore, we performed the immunostaining of the specimen. The centrally located chief cells of paraganglioma prove by immunohistochemistry to be NSE positive, choromogranin positive and neurofilament positive, they are S-100 protein negative [8]. S-100 protein was positive indicating a neural tumour. S-100 protein demonstrates neuroectodermal origin of tumours [9]. In this case, S-100 protein showed a positive reaction, but negative results were obtained for NSE, chromogranin and neurofilament. These findings excluded a diagnosis of paraganglioma, and gave us the final diagnosis of neurilemmoma.
Conclusion
We have reported a case of vagal neurilemmoma closely resembling large paraganglioma on MRI. Transcervical-transmandibular approach was useful in achieving total extirpation of the large tumour in the poststyloid parapharyngeal space, providing the necessary wide, clear surgical fields and avoiding complications in the surgical procedure. Immunohistochemistry provided a definitive diagnosis of neurilemmoma.
Conflict of Interests
The authors declare that there are no conflicts of interests with regard to the publication of this paper.
Financial or Other Competing Interests
None.
[1]. Whitaker WG, Droulias C, Benign encapsulated neurilemoma: A report of 76 casesAm Surg 1976 42(9):675-78. [Google Scholar]
[2]. Katz AD, Passy V, Kaplan L, Neurogenous neoplasms of major nerves of face and neckArch Surg 1971 103(1):51-56. [Google Scholar]
[3]. Urquhart AC, Johnson JT, Myers EN, Schechter GL, Glomus vagale: paraganglioma of the vagus nerveLaryngoscope 1994 104(4):440-45. [Google Scholar]
[4]. Lin J, Martel W, Cross-sectional imaging of peripheral nerve sheath tumours: characteristics signs on CT, MR imaging, and sonographyAm J Roentgenol 2001 176(1):75-82. [Google Scholar]
[5]. Olsen WL, Dillon WP, Kelly WM, Norman D, Brant-Zawadzki M, Newton TH, MR imaging of paragangliomasAm J Roentgenol 1987 148(1):201-04. [Google Scholar]
[6]. Frank RM, John RW, Pierre L, Benjamin GW, Magnetic resonance imaging and the management of parapharyngeal space tumoursHead and Neck 1996 18(1):67-77. [Google Scholar]
[7]. Browne JD, Fisch U, Valavanis A, Surgical therapy of glomus vagale tumoursSkull Base Surg 1993 3(4):182-92. [Google Scholar]
[8]. Pellitteri PK, Rinald A, Myssiorek D, Gary Jackson C, Bradly PJ, Devaney KO, Paragangliomas of the head and neckOral Oncology 2004 40(6):563-75. [Google Scholar]
[9]. Budu VA, Bulescu IA, Popp CG, Mocanu BC, Mogoanta CA, Vagus nerve schwannoma in the parapharyngeal space: surgical, histological and immunohistochemical aspects. A case reportRom J Morphol Embryol 2015 56(1):273-76. [Google Scholar]