Glaucoma is a chronic, progressive optic neuropathy caused by a group of ocular conditions which lead to damage of the optic nerve with loss of visual function. It is the second leading cause of blindness [1]. The prevalence of POAG according to population based studies ranges from 0.4 to 8.8% [2]. Intraocular pressure (IOP) is found to be the single most important risk factor for the development of glaucomatous optic neuropathy, and hence its measurement is vital in the initial diagnosis and management of the glaucomas [3]. Amongst the multitude of options available for medical treatment of elevated IOP, topical beta blockers and the prostaglandin F2α analogue Latanoprost are the most commonly prescribed medications [4]. Timolol, a beta-adrenergic blocking agent, reduces the intraocular pressure by reducing aqueous formation [5]. Latanoprost, a prostaglandin analogue has come up with powerful ocular hypotensive effects [6]. The objective of present study was to compare the IOP lowering efficacy of Latanoprost with Timolol in patients with primary open angle glaucoma (POAG).
Materials and Methods
This was a prospective, randomized study conducted at one of the tertiary health care centre in Calicut, India.
Inclusion Criteria
Patients aged 40 years or older.
Patients with a diagnosis of primary open angle glaucoma.
Patients with baseline IOP (after washout) more than 21 mmHg in each eye.
Exclusion Criteria
Active ocular disease.
Significant hypersensitivity to prostaglandin and it’s analogue, topical or systemic β-blocker.
Use of other ocular medications or other therapies that might have substantial effect on IOP.
Ocular surgeries in past 3 months.
Ocular inflammation and infection within past three months.
Ocular trauma within past six months.
Glaucomas other than POAG.
Pregnancy and lactation.
The enrolled patients were randomly divided into two groups by block randomization. The sample size was determined to be 60 in each group using the formula:
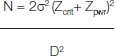
N – Sample size in each group.
σ – Assumed SD of each group.
Zcrit – Desired significance criterion.
Zpwr – Desired statistical power.
D - Minimum expected difference between the two means.
The study was conducted over a period of two years from Jan 2012 to Dec 2013. Institutional Ethical committee approval was taken and No. is-8-30/11/11. Written informed consent was obtained from the patient before starting the study. Baseline IOP was measured after washout period of one week in the patients fulfilling the study criteria. Patients were randomly assigned to one of the two treatment groups i.e. 0.005% latanoprost ophthalmic solution once daily in the evening or 0.5% timolol maleate twice daily. Patients were given their medications after all evaluations at the baseline visit. Patients were followed up at 2 weeks, 4weeks, 6 weeks and 3 months after baseline visit. Applanation tonometer was used to measure IOP at each visit.
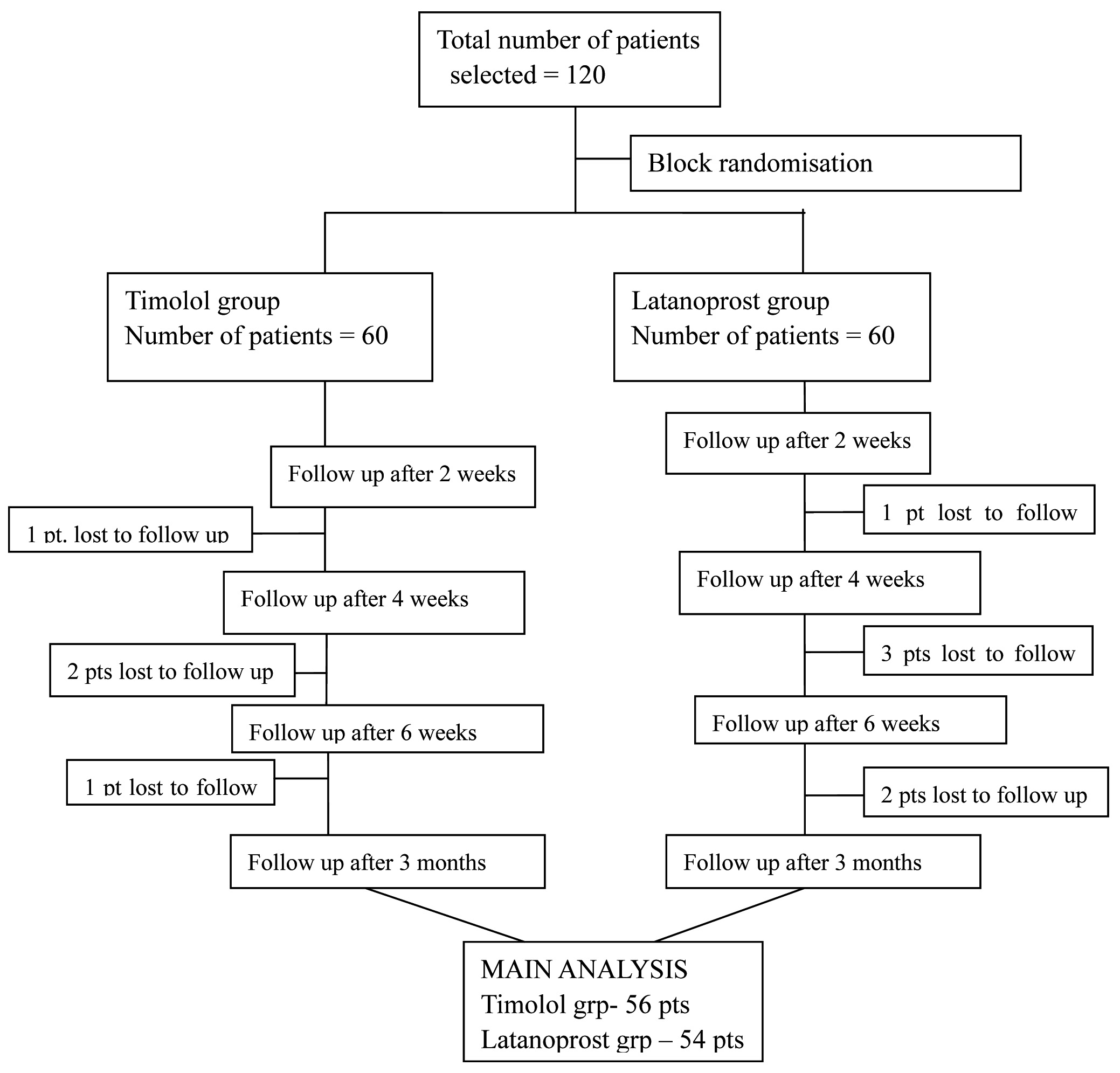
Patients attending the glaucoma clinic were screened and 120 patients were chosen among them, who fulfilled the selection criteria.
Statistical Analysis
Statistical analysis was done using Statistical package for social service (SPSS) software. Statistical analysis was performed on patients who had completed the 12 weeks of treatment period. Using independent sample test baseline parameters like age and intraocular pressure of the two groups were compared. Drug effect in terms of change in IOP was compared using Independent sample test. The p-value of less than 0.05 was considered statistically significant.
Results
A total of 120 patients were enrolled in the study, out of which 56 patients in Timolol group and 54 patients in Latanoprost group completed the study. Rest were lost to follow up [Table/Fig-1].
In the Timolol group, the mean baseline IOP was 23.7±2.21 and in the Latanoprost group, the mean baseline IOP was 24.26±1.99. Both the groups are comparable [Table/Fig-2,3].
Comparison of baseline parameters.
| Characteristics | Timolol (n=56) | Latanoprost (n=54) |
|---|
| Number of patients included in the study | 60 | 60 |
| Number of patients completed the study | 56 | 54 |
| Males | 24 (42.9 %) | 34 (62.94 %) |
| Females | 32 (57.14 %) | 20 (37.04%) |
| Mean age | 55.29 ± 8.645 | 59.07 ± 8.901 |
| Mean baseline IOP | 23.7 ± 2.21 | 24.26 ± 1.99 |
Comparison of baseline clinical features.
| Clinical features | Timolol | Latanoprost |
|---|
| n | % | n | % |
|---|
| Headache | 30 | 53.57 | 20 | 37.04 |
| Blurring of vision | 24 | 42.86 | 32 | 59.26 |
| Frequent change of glass | 10 | 17.86 | 16 | 29.63 |
| Visual field defect | 4 | 7.14 | 6 | 11.11 |
| Others | 8 | 14.29 | 6 | 11.11 |
Patients in both the groups showed reduction in IOP from 0 weeks towards the end of 3 months. The mean IOP at 3 months in Timolol group was 16.43±2.206 and in Latanoprost group was 14.54 ± 2.8. In comparison to Timolol, the Latanoprost group showed a better reduction in IOP which was statistically significant with a p-value <0.05 [Table/Fig-4].
Mean IOP in each group recorded at each follow up visit.
| Visit | Mean IOP ± SD in two groups | t-value | p-value |
|---|
| Timolol | Latanoprost |
|---|
| 0wk | 23.7 ± 2.21 | 24.26 ± 1.99 | -1.414 | 0.160 |
| 2wk | 18.94 ± 3.43 | 17.22 ± 2.15 | 3.16 | 0.002 |
| 4wk | 17.66 ± 1.98 | 15.65 ± 2.03 | 5.259 | < 0.001 |
| 6wk | 17.43 ± 2.6 | 14.83 ± 2.3 | 5.587 | < 0.001 |
| 3month | 16.43 ± 2.206 | 14.54 ± 2.78 | 3.953 | < 0.001 |
Statistical analysis of mean reduction in IOP was done using Independent samples test. In the Timolol group the mean reduction in IOP at 3 months was 7.27±3.1 and in the Latanoprost group it was 9.72±2.435. The mean reduction from the baseline IOP at each follow up visit was significantly greater in the Latanoprost group as compared with Timolol group. The p value was <0.05 in all the follow up visits [Table/Fig-5].
Mean reduction in IOP from baseline.
| Visit week | Mean reduction in IOP±SD in two groups | t-value | p-value |
|---|
| Timolol | Latanoprost |
|---|
| 2 weeks | 4.75 ± 3.08 | 7.04 ± 2.9 | -4.025 | <0.001 |
| 4 weeks | 6.04 ± 3.05 | 8.61 ± 2.025 | -5.196 | < 0.001 |
| 6 weeks | 6.27 ± 3.1 | 9.43 ± 1.99 | -6.35 | <0.001 |
| 3 months | 7.27 ± 3.1 | 9.72 ± 2.435 | -4.607 | < 0.001 |
Discussion
Glaucoma is a potentially blinding ocular disease having multiple causes. Raised intraocular pressure is a significant and modifiable risk factor in the development and progression of Glaucoma [7]. This disease is often insidious in onset and gradually progressive resulting in permanent visual loss. Hence, it is also called as the “silent thief of sight” [8]. Primary open-angle glaucoma is an enigma which involves abnormal aqueous outflow [9]. Many randomized clinical trials have shown that reducing intraocular pressure slows the onset and progression of glaucoma [10,11].
Age is recognised as a risk factor in glaucoma [12]. In our study, in the Timolol group, the mean age was 55 years and in the Latanoprost group, the mean age was 59 years [Table/Fig-2].
The Framingham eye study showed that the prevalence was twice in men than women. In Sweden, woman showed higher frequency of POAG. Many other studies revealed no difference[13]. In our study, in the Timolol group, 43% of the patients were male and 57% were females. In the Latanoprost group 63% of the patients were males and 37% were females.
Majority of the patients in both the groups had headache and blurring of vision as the presenting symptom. Other clinical features noted were frequent change of glass, visual field defect and ocular pain [Table/Fig-3].
Previous research has demonstrated that progression of glaucoma or ocular hypertension can be delayed or halted by lowering IOP levels through the use of ocular hypotensive agents [14].
Till recently, beta blockers administered twice a day was considered the first choice treatment in decreasing the raised intraocular pressure [15]. But now Latanoprost, the first prostaglandin F2 analogue to be introduced in the treatment of open angle glaucoma, has become the most commonly prescribed glaucoma therapy in adults [16].
Gulati V et al., conducted a double masked, randomised, cross over study to evaluate the interaction of antiglaucoma drugs with physiologic day and night changes in aqueous humor dynamics in ocular hypertensive patients [17]. It showed that Latanoprost is better than Timolol in reducing night time intraocular pressure.
Several studies have proven the efficacy of Latanoprost over Timolol. Study by Osude-Uzodike & Anthony showed that 0.005% latanoprost ophthalmic sterile solution was found to be more effective in IOP reduction and better tolerated by the patients than 0.5% timolol maleate sterile ophthalmic solution, hence can be used by practitioners as drug of choice for management of open angle glaucoma [18].
A study conducted by Harasymowycz P et al., to compare Latanoprost and Timolol- gel forming solution once daily in POAG showed that Latanoprost reduced IOP more than Timolol. Also, switching to Latanoprost monotherapy caused further IOP reduction in patients who initially did not respond to Timolol [19].
A metaanalysis done by Hedman showed Latanoprost to produce statistically significant reduction in mean IOP from the baseline. Also, the number of patients reaching the target IOP was more in Latanoprost group as compared to Timolol group. These findings were supported by another meta analysis done by Zhang [20].
Timolol, a beta blocker has to be administered twice daily whereas once daily administration is sufficient for Latanoprost. Hence, better patient compliance. The present study was undertaken to compare the efficacy of twice daily 0.5% Timolol and once daily 0.005% Latanoprost in patients with POAG [Table/Fig-4]. In our study, patients in both the groups showed reduction in IOP from baseline towards the end of 3 months. The improved efficacy of Latanoprost was shown by significantly greater reduction of mean IOP from the baseline to the end of the study [Table/Fig-5]. This data was similar to other clinical studies [4,18,20]. Results of the present study suggest that 0.005% Latanoprost once daily is superior in efficacy compared to 0.5% Timolol twice daily in the treatment of glaucoma as assessed by reduction of IOP.
Limitations
As the present study included a small number of patients within a limited period of time, further studies with large number of patients have to be carried out to establish the data. Also, since it was a short term study, the effect of drugs on visual field defect cannot be determined properly. In addition, since the study was not double blinded, the chance of bias cannot be excluded.
Conclusion
Once daily 0.005% Latanoprost therapy provides significant IOP lowering effect which is superior to twice daily 0.5% Timolol therapy, in patients with primary open angle glaucoma. Latanoprost is more potent and efficacious when compared to Timolol. Also, it needs to be administered once daily unlike Timolol which needs twice daily administration. Hence, patient compliance is better with Latanoprost.
Limitations
As the present study included a small number of patients within a limited period of time, further studies with large number of patients have to be carried out to establish the data. Also, since it was a short term study, the effect of drugs on visual field defect cannot be determined properly. In addition, since the study was not double blinded, the chance of bias cannot be excluded.