Severe Hypokalaemia, Hypertension, and Intestinal Perforation in Ectopic Adrenocorticotropic Hormone Syndrome
Tezcan Kaya1, Cengiz Karacaer2, Seyyid Bilal Açikgöz3, Yusuf Aydemir4, Ali Tamer5
1 Assistant Professor, Department of Internal Medicine, Sakarya University Faculty of Medicine, Sakarya, Turkey.
2 Medical Doctor, Internal Medicine Specialist, Department of Internal Medicine, Sakarya University Training and Research Hospital, Sakarya, Turkey.
3 Medical Doctor, Research Assistant, Department of Internal Medicine, Sakarya University Faculty of Medicine, Sakarya, Turkey.
4 Assistant Professor, Department of Chest Diseases, Sakarya University Faculty of Medicine, Sakarya, Turkey.
5 Professor, Department of Internal Medicine, Sakarya University Faculty of Medicine, Sakarya, Turkey.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Tezcan Kaya, Sakarya University Faculty of Medicine, Department of Internal Medicine, 54100 Sakarya, Turkey.
E-mail: tezcankaya@gmail.com
Ectopic adrenocorticotropic hormone (ACTH) syndrome is a rare cause of the Cushing’s syndrome. The occurrence of the ectopic ACTH syndrome presenting with severe hypokalaemia, metabolic alkalosis, and hypertension has been highlighted in case reports. However, presentation with lower gastrointestinal perforation is not known. We report the case of a 70-year-old male patient with severe hypokalaemia, metabolic alkalosis, hypertension, and colonic perforation as manifestations of an ACTH-secreting small cell lung carcinoma. Ectopic ACTH syndrome should be kept in mind as a cause of hypokalaemia, hypertension, and intestinal perforation in patients with lung carcinoma.
Cushing’s syndrome, Hypercortisolism, Lung carcinoma
Case Report
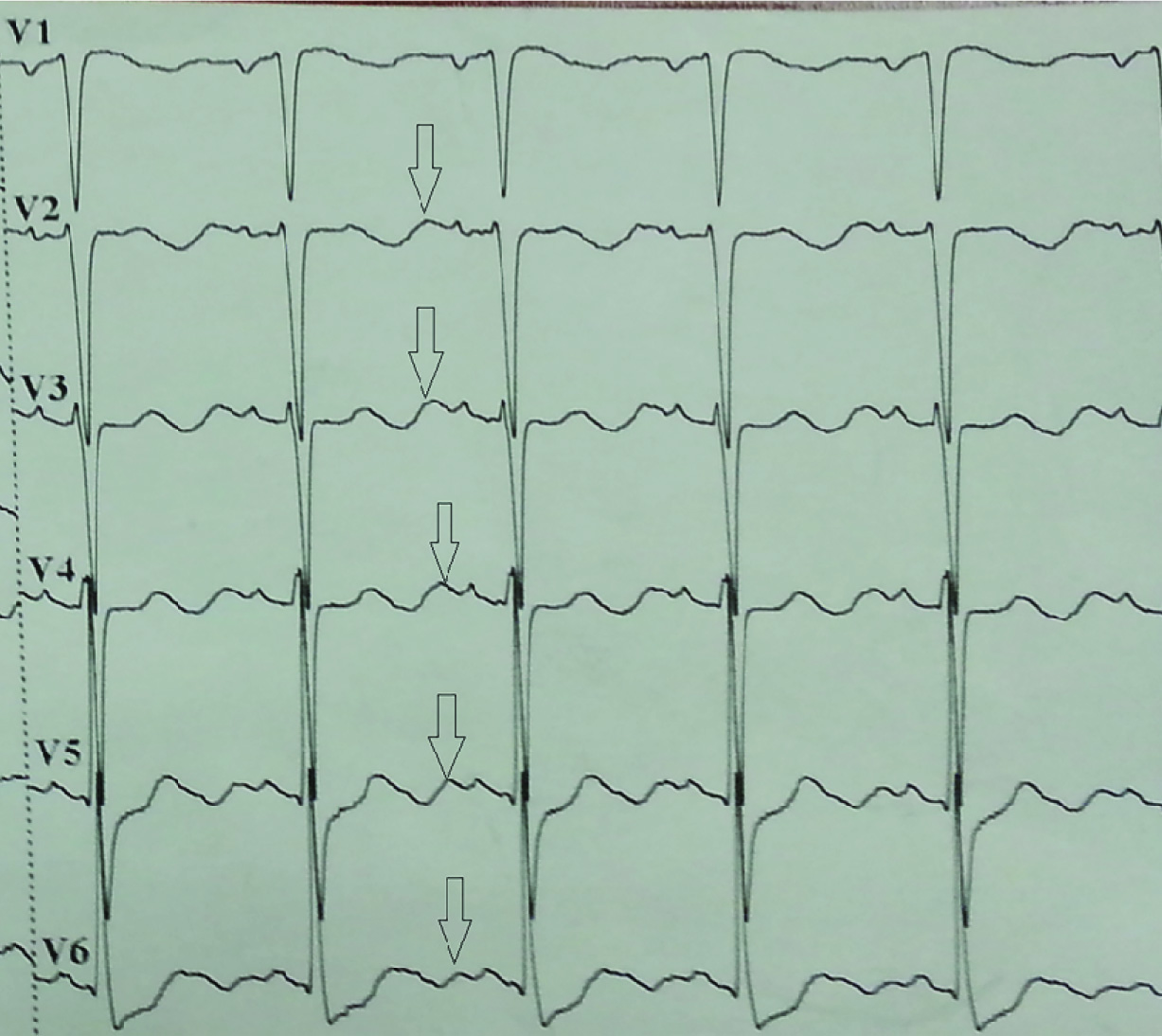
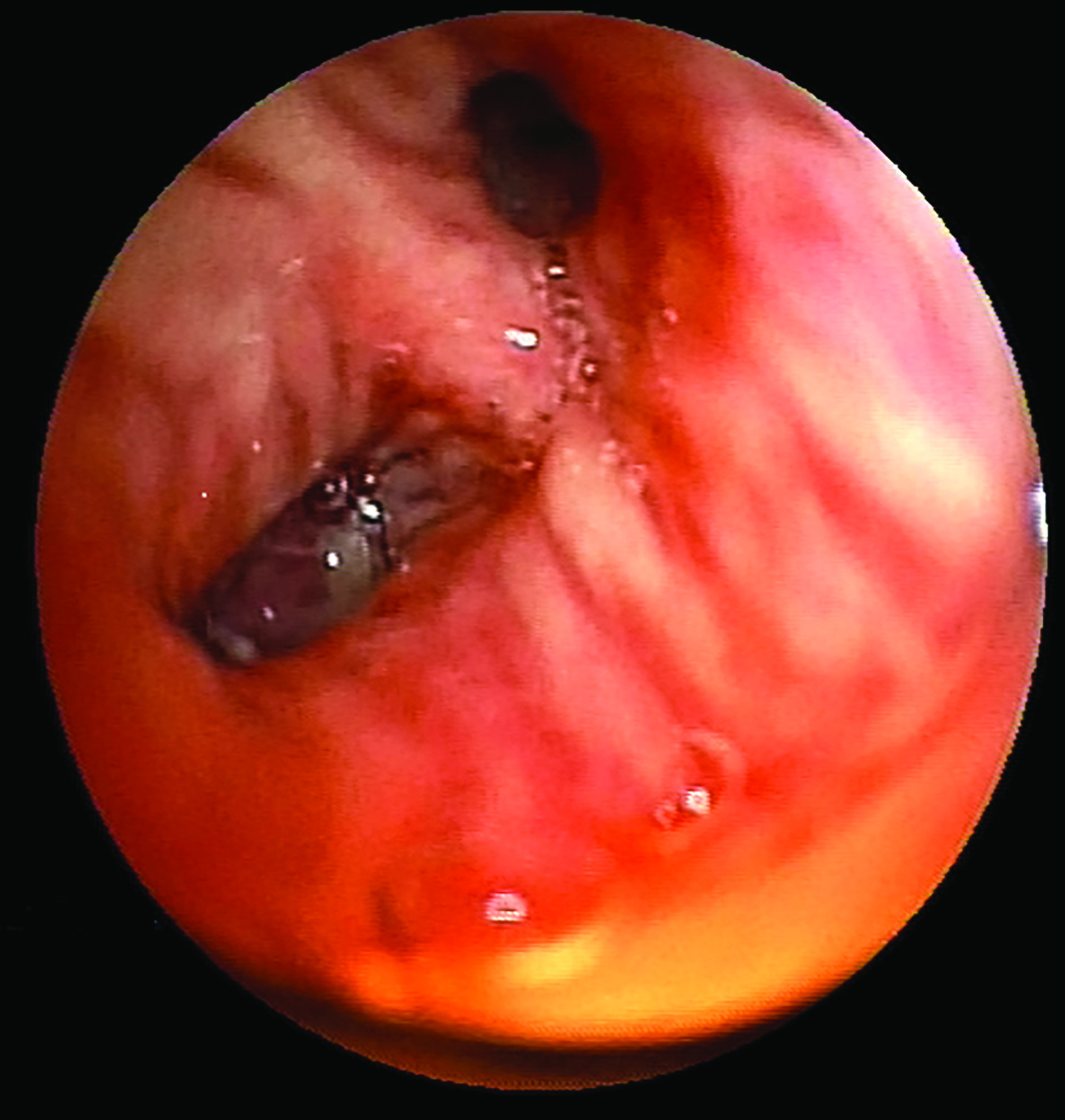
A 70-year-old man was admitted to the emergency department of our hospital with weakness, inappetence, and jaundice. He had these complaints for approximately 15 days and he was unable to walk because of his symptoms and condition. He had been using ramipril and hydrochlorothiazide, owing to hypertension for approximately 10 years. He was a 15-pack-year smoker for 40 years. On examination, he was oriented, co-operative, and afebrile. Blood pressure was 180/95 mmHg, pulse rate 80 beats/min, and respiratory rate 20 breaths/min. He was icteric and muscle strength was graded as 3/5 in all the extremities. Laboratory results were as follows: urea 101 mg/dL; creatinine 1.1 mg/dl; uric acid 13.4 mg/dL; calcium 8.7 mg/dL; phosphorus 2.7 mg/dL; AST 160 U/L; ALT 133 U/L; lactic dehydrogenase 1284 U/L; amylase 84 U/L; total bilirubin 5.8 mg/dL; direct bilirubin 5.2 mg/dL; albumin 3 g/dL; sodium 145; chloride 74 mmol/L; potassium 1.6 mmol/L; prothrombine time 10.4 s; INR 0.97; haemoglobin 14.3 gr/dl; white cell count 12900 K/uL; and thrombocyte 265000 K/uL. Viral hepatitis markers were negative. The blood gas analysis was indicative of metabolic alkalosis with a pH of 7.52, PaCO2 of 37.1, and HCO3 of 38.1. Electrocardiogram revealed a normal sinus rhythm with prominent U waves [Table/Fig-1]. The patient was hospitalized and was given parenteral potassium replacement. Amlodipine and spironolactone were administered for high blood pressure. There were no pathological findings in the chest CT other than pre-tracheal, pre-carinal, and left hilar multiple lymph nodes with diameters up to 21 mm. Abdominal CT revealed multiple metastatic lesions in the liver, multiple para-aortic lymphadenopathies, and colonic diverticulosis [Table/Fig-2]. Upper and lower gastrointestinal endoscopies were normal except for the presence of colonic diverticulosis. Because of the hypokalaemia and hypertension, we investigated endocrine hypertension. Adrenocorticotropic hormone (ACTH) was 112 pg/mL (normal range<50), plasma renin was 16.1 pg/mL (normal range 5-27.8), aldosterone was 115 pg/mL (normal range 30-162), and cortisol was 51.9 μg/dL (normal range 3.7-19.4). No suppression was observed in cortisol levels with the 8-mg dexamethasone suppression test. Magnetic resonance imaging of the pituitary gland was normal. These results together with the biochemical, clinical, and radiological findings supported the diagnostic hypothesis of malignant origin ectopic ACTH syndrome (EAS). We initiated oral ketoconazole treatment for the hypercortisolism. After administering ketoconazole, the patient’s need for potassium decreased and blood pressure returned to normal levels. Bronchoscopy showed an obstructing mass in the superior sub segment of the lower lobe of the left lung and a biopsy indicated small cell lung carcinoma (SCLC) [Table/Fig-3,4]. Immunohistochemical analysis of biopsy was positive for ACTH. During the follow-up, an acute abdomen developed and an abdominal CT scan showed free air and free fluid within the abdomen. The patient underwent surgery for intestinal perforation that was thought to result from diverticulitis in the rectosigmoid area. Postoperatively, he was supported with mechanical ventilation while being followed up in the intensive care unit. On the twelfth hospital day and the first postoperative day the patient died.
Electrocardiogram of the patient showing prominent U wave (arrow) as typical feature of severe hypokalaemia
Abdominal computed tomography scan showing metastatic lesions in the liver

Bronchoscopic examination shows an intra-bronchial tumour in superior segmental bronchus of left lower lung
Pathohistological diagnosis of bronchoscopic biopsy revealed small cell carcinoma of lung

Discussion
EAS is defined as a clinical syndrome of hypercortisolism caused by non-pituitary ACTH-secreting tumour [1]. EAS is a rare cause of the Cushing’s syndrome, seen in approximately 10% of cases [1,2]. The most frequent causes of EAS are carcinoid tumour, thymic carcinoma, neuroendocrine tumour, small cell lung carcinoma, thyroid medullary carcinoma, pancreas islet cell tumour, and pheochromocytoma [1,2]. Hypokalaemia and hypertension because of EAS are rarely encountered and have been described in case presentations. To the best of our knowledge, only two cases of intestinal perforation as a presenting clinical symptom of Cushing’s disease were noted [3,4]. However, lower gastrointestinal perforation in EAS has not yet been reported. We report a patient with severe hypokalaemia, hypertension, and colonic diverticular perforation as manifestations of EAS because of SCLC.
Most of the ACTH-dependent Cushing’s syndrome cases are Cushing’s disease caused by pituitary microadenomas [2]. In some cases, differentiating between Cushing’s disease and EAS may be difficult. Parameters that can be used for differential diagnosis are plasma ACTH level, plasma potassium level, high-dose dexamethasone suppression test, metyrapone test, corticotrophin-releasing hormone test, inferior petrosal sinus sampling, and pituitary MR imaging [1]. Morning plasma ACTH levels in ectopic Cushing’s syndrome are increased more than in Cushing’s disease. Morning plasma ACTH levels in 50% of Cushing’s disease cases are within normal limits, and slightly high in the rest of the cases [5,6]. It is usually higher than 90 pg/mL in EAS [5,6]. While hypokalemic alkalosis exists in more than 95% of EAS, in Cushing’s disease it is encountered in less than 10% [5,6]. Approximately 90% of Cushing’s disease patients have a positive response to the high-dose dexamethasone (4 × 2 mg/day, 48 h) suppression test, but there is such a response in around 10% of EAS cases [5,6]. Bilateral inferior petrosal sinus sampling is considered to be the gold standard in establishing a diagnosis of EAS [1]. In this patient, most of the above mentioned parameters were in favor of a diagnosis of EAS, and the presence of a malignancy that could cause EAS supported the diagnostic hypothesis of ectopic ACTH-dependent Cushing’s syndrome.
Prevalence of colonic diverticulosis increases with age [7], whereas the prevalence in people under the age of 40 years is 16%–22%, and it is 42%–60% in people above the age of 80 years [7]. However, most of the diverticulosis cases remain asymptomatic lifelong; symptoms and findings appear only in 10%–25% of the cases [8]. Although diverticulitis regresses with medical treatment in many cases, it may cause abscess, obstruction, perforation or internal fistulization in some patients [7,8]. To the best of our knowledge, this is the first published case of lower intestinal perforation as a clinical symptom of EAS. Stress, metastasis of malignancy, excessive secretion of gastrointestinal fluid, diverticulitis, and gastrointestinal tumours may lead to intestinal perforation in patients with diverticulosis [8]. On the other hand, corticosteroid excess may prone to collagen damage and easy injury [9,10]. The lamina propria of large intestine mucosa is consists of a thick layer of collagen and ground substance. Since collagen protein is a major component of lamina propria of large intestine, decreased proportion of this protein should cause a reduction in intestine fold thickness [9,10]. In this context, increased endogenous glucocorticoid because of EAS may have contributed to the bowel perforation by causing tissue fragility. But the association between diverticular perforation and hypercortisolism is not exactly known [3,4].
The tumour type determines the prognosis of the EAS patient and the main treatment is the surgical removal of the ACTH-secreting tumour [1]. Metyrapone, ketoconazole, and glucocorticoid synthesis inhibitors can be used for the treatment of hypercortisolaemia [1,2]. Moreover, bilateral adrenalectomy can be applied to the patients whose hypercortisolism continues or to those who have occult tumours, despite malignancy treatment [1]. Patients with Cushing’s syndrome and SCLC appear to have a worse prognosis than patients with SCLC without Cushing’s syndrome [11].
Conclusion
We described, a case of SCLC who presented with severe hypokalaemia, hypertension, and colonic perforation because of EAS. This should be kept in mind as a cause of hypokalaemia, hypertension, and intestinal perforation in patients with malignancy.
[1]. Isidori AM, Lenzi A, Ectopic ACTH syndromeArq Bras Endocrinol Metabol 2007 51(8):1217-25. [Google Scholar]
[2]. Newell-Price J, Bertagna X, Grossman AB, Nieman LK, Cushing syndromeLancet 2006 367(9522):1605-17. [Google Scholar]
[3]. Hara T, Akutsu H, Yamamoto T, Ishikawa E, Matsuda M, Matsumura A, Cushing’s disease presenting with gastrointestinal perforation: a case reportEndocrinol Diabetes Metab Case Rep 2013 :130064DOI: 10.1530/EDM-13-0064 [Google Scholar]
[4]. De Havenon A, Ehrenkranz J, A perforated diverticulum in Cushing’s diseaseInt J Surg Case Rep 2011 2(7):215-17. [Google Scholar]
[5]. Guignat L, Berherat J, The Diagnosis of Cushing’ syndrome: an Endocrine Society Clinical Practice Guideline: commentary from a European perspectiveEur J Endocrinol 2010 163(1):9-13. [Google Scholar]
[6]. Lacroix A, Feelders RA, Stratakis CA, Nieman LK, Cushing’s syndromeLancet 2015 386(9996):913-27.doi: 10.1016/S0140-6736(14)61375-1 [Google Scholar]
[7]. Stollman N, Raskin JB, Diverticular disease of the colonLancet 2004 363(9409):631-39. [Google Scholar]
[8]. Parra-Blanco A, Colonic diverticular disease: pathophysiology and clinical pictureDigestion 2006 73(Suppl 1):47-57. [Google Scholar]
[9]. Rokowski K, Sheehy J, Cutroneo KR, Glucocorticoid-mediated selective reduction of functioning collagen messenger ribonucleic acidArch Biochem Biophys 1981 210:74-81. [Google Scholar]
[10]. Yanovski JA, Cutler GB, Glucocorticoid action and the clinical features of Cushing’s syndromeEndocrinol Metab Clin North Am 1994 23:487-509. [Google Scholar]
[11]. Shepherd FA, Laskey J, Evans WK, Goss PE, Johansen E, Khamsi F, Cushing’s syndrome associated with ectopic corticotropin production and small-cell lung cancerJ Clin Oncol 1992 10(1):21-27. [Google Scholar]