Introduction
Injuries and violence are one of the leading causes of mortality worldwide. More than nine people die every minute from injuries according to the World Health Organisation [1]. A substantial portion of these injuries involve the maxillofacial region. Moreover maxillofacial trauma itself may be associated with significant injuries elsewhere [2]. Among the concomitant injuries, injury to the head and cervical spine (C-spine) are amongst those that demand due consideration on account of their life threatening behaviour [3].
Head injury can be divided into 3 groups [4] as illustrated in [Table/Fig-1]. Traumatic brain injury (TBI) is defined “as evidence of loss of consciousness and / or post-traumatic amnesia in a patient with a non-penetrating head injury” [5].
Classification of head injury.
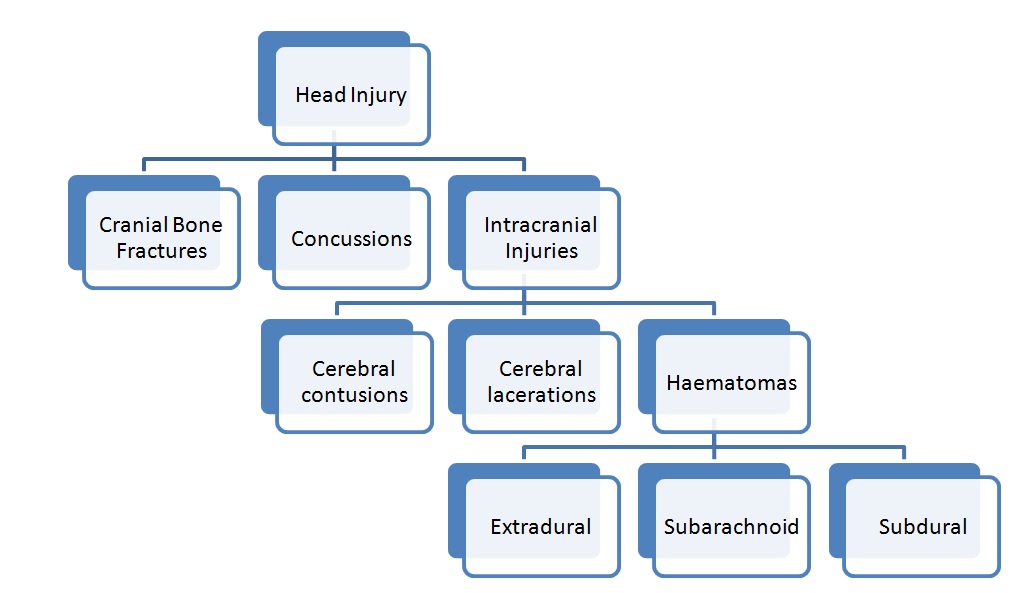
Head injury may be either primary or secondary in nature. Release of biochemical substances during a primary injury causes neural damage thus causing a secondary injury. Secondary injury also occurs due to hypotension or hypoxia [6].
As opposed to injuries elsewhere in the body, brain injury is often difficult to predict initially. This is because the brain is enveloped in a rigid bony structure. The injury to the brain may be severe irrespective of whether the calvarium is intact or not. The severity of the brain injury will thus depend on the structural damage of the brain tissue and the extent of the haemorrhage. Early recognition of symptoms of intracranial damage is hence imperative [7].
Therefore as maxillofacial surgeons, we must be aware of the possible concomitant head injury. We must also have a thorough understanding of the pathophysiology of head trauma for initial recognition and management before the arrival of the neurosurgeon.
Association between Maxillofacial Trauma and Head injury: According to Keenan et al., patients with maxillofacial fractures have a higher risk of intracranial haemorrhage when compared to patients without maxillofacial fractures due to trauma [8]. A study done by Haug and associates revealed that 17.5% patients with facial fractures had some form of closed head injury whereas almost 10% sustained a severe intracranial injury [9]. A ten year analysis of traumatic maxillofacial and brain injury patients by Erik and associates suggest that maxillofacial trauma does have an association with traumatic brain injury that requires neurosurgical intervention, with an incidence rate of 8.1% [5].
The predictors of intracranial haemorrhage include vomiting/nausea, skull fractures, seizures and C-spine injury. Among these C-spine injury is the best predictor of intra cranial haemorrhage. Vomiting is linked with a 25% higher risk of intracranial haemorrhage and seizures are linked with a 15% higher risk of intracranial haemorrhage [10].
Many times, facial fractures tend to distract our attention from more severe and often life threatening injuries [11]. Usually conscious patients with a Glasgow Coma Scale (GCS) score of 15 with no clinical neurological abnormalities are not expected to have an intracranial pathology. However, high velocity impact can result in intracranial haemorrhage. 2.8% of neurologically “normal” patients suffer from intracranial haematomas [10]. Hence intracranial haemorrhage cannot be excluded in these patients. Early diagnosis of these intracranial haemorrhage leads to prompt treatment which is essential to improve the outcome of these patients [12].
Any patient with maxillofacial injury irrespective of whether it is associated with fractures or not is always at risk of traumatic brain injury. Hence, all the patients with maxillofacial injuries should be under neurosurgical observation and regular Follow up [13].
Initial Assessment: It is the duty of a maxillofacial surgeon to carry out a systematic initial assessment without focussing solely on maxillofacial injuries sustained by the patient. For the initial assessment of these patients, the protocol devised by the American College of Surgeons, known as Advanced Trauma Life Support (ATLS) is widely used [14]. A brief description of the initial assessment of trauma patient is described in a nutshell in [Table/Fig-2]. We will also deal with certain special considerations to be kept in mind while treating patients with head injury.
Principles of ATLS approach for initial assessment of a trauma patient.
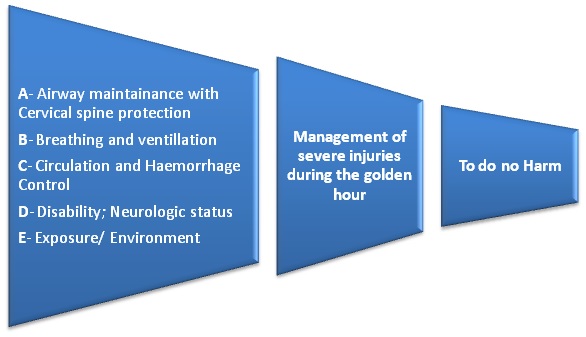
Airway Maintanance: There exists a threat to airway in the presence of maxillofacial trauma. Foreign bodies, blood and vomitus are commonly known to obstruct the airway [15]. Apart from these, retropharyngeal haematoma may also cause a delayed airway compromise [16]. Presence of retropharyngeal haematoma often indicates the presence of C-spine injury [16].
As a maxillofacial surgeon, what steps can we take to stabilise the airway before the anaesthetist arrives?
High flow oxygen is given at15 litres/min.
A jaw thrust should be performed.
Chin lift or head tilt should be avoided if in case C-spine injury is suspected.
An oropharyngeal airway is considered if the GCS<8.
A nasopharyngeal airway should be avoided.
If the patient is not breathing spontaneously but the airway is patent, manual ventilation should be provided with a self-inflating bag and mask.
A laryngeal mask airway (LMA) should be introduced if GCS<8 and jaw thrust and manual ventilation are proving to be ineffective.
Orotracheal intubation should be attempted only if one is confident of it, otherwise a cricothyroidotomy should be performed and the anaesthetist called for [15].
We should remember the dictum “Keep it simple- if intubation is not a skill you commonly perform, it is best not to attempt it” [15].
To decrease the risk of raising the intracranial pressure (ICP) in head injury patients and to avoid secondary brain injury, Rapid Sequence Intubation is recommended. Following precautions are needed while intubating a patient with head injury [17]:
Positioning the patient in reverse Trendelenburg position at the time of intubation and immediately after intubation.
Prevention of sympathetic response during intubation by liberal use of muscle relaxants and analgesics or sedatives.
Avoiding aspiration.
Avoiding hypoventilation as it can increase the intracranial pressure and hyperventilation as it may cause an increase in cerebral vasoconstriction.
Avoiding hypoxaemia as it increases cerebral injury.
Maintaining moderate hyperoxaemia (PaO2 110–300 mmHg).
Maintaining cerebral perfusion pressure (CPP) > 60 mmHg.
Intubating the patient in the early phase has its own pros and cons. Apart from securing the airway, intubation also allows one to perform certain emergency management procedures such as chest drain placement more easily. In the absence of swallowing in the intubated patient, maxillofacial bleeding can be identified readily. However, intubating the patient results in a loss of response at an early stage. This disables the practitioner to assess the neurologic status and other injuries. Anaesthesia results in a change in the haemodynamic status of the patient which may complicate the general condition of the patient which is already compromised under the influence of trauma. If the patient is not intubated and the airway is maintained, it allows repeated clinical assessment, however at the risk of compromising the airway, in the event that the patient vomits. Also, progressive facial swelling can make intubation difficult at a later stage [15].
Presence of head injury and facial injuries increases the likelihood of c-spine injury [18]. Also, it has been suggested that patients with a GCS score below 8 have higher chances of sustaining a C-spine injury [19]. ATLS suggests a complete immobilization of the patient with semi rigid collars, straps, tapes and backboard until the C-spine is cleared [14].
Further, cervical movement during examination and initial airway management can be prevented more effectively using Manual in-line immobilization technique [17]. In patients with GCS<8, C-spine should always be treated like it is injured, until proven otherwise. Ligament injuries can be missed even with a computed tomography (CT) scan. Magnetic resonance imaging (MRI) is the gold standard for detecting injured ligaments and spinal cord [15]. The role of dynamic fluoroscopy has been suggested in screening the stability of C-spine in unconscious patients [20].
The following factors can rule out the C-spine injury or more appropriately can “clear the C-spine” [21].
Awake patients with a GCS score of 14 or above.
Patient not under any intoxication (e.g. alcohol).
No distracting injuries which can drive one’s attention away from the C- spine.
Absence of tenderness on palpation of spine.
Absence of pain during neck movements.
We must also be prepared to manage unexpected vomiting in the patient as this poses one of the major risks to airway. Blockage of the airway due to vomitus can be prevented by bringing the head end of the trolley at an inferior level along with thorough suctioning [15].
Assessment of Level of Conciousness
Glasgow Coma Scale: [Table/Fig-3]
| EYE OPENING | SCORE |
|---|
| Spontaneous | 4 |
| To voice | 3 |
| To pain | 2 |
| None | 1 |
| VERBAL RESPONSE | SCORE |
| Oriented, normal conversation | 5 |
| Confused conversation | 4 |
| Words but not coherent | 3 |
| Incomprehensible sounds | 2 |
| None | 1 |
| MOTOR RESPONSE | SCORE |
| Obeys commands | 6 |
| Localizes to pain | 5 |
| Withdraws to pain | 4 |
| Abnormal flexion(decorticate posture) | 3 |
| Abnormal extension(decerebrate posture) | 2 |
| None | 1 |
Glasgow Coma Scale is the most commonly used tool to assess the level of consciousness among the others. It is given by Jennet and Teasdale in 1974. The present 15 point scale was devised in 1976 [22]. It is used to assess the level of consciousness, aids in therapeutic and diagnostic decision making and also to assess the prognosis of TBI [23]. The main disadvantage of GCS is interpersonal variability in assessment of scores which may lead to unnecessary neurosurgical interventions [24]. To overcome this disadvantage, one must repeat the assessment multiple times at various intervals. GCS does not differentiate causes of altered mental status. The most reliable GCS score will be the one which was assessed after resuscitation. The motor component of GCS alone is as sensitive as the total GCS score in predicting the severity of head injury [25]. The association between TBI severity and GCS score is affected by age. In patients with similar TBI severity, the older patients have better GCS scores [26].
Investigations
Computed Tomography (CT): In head injury patients, CT is the imaging modality of choice [27]. It is most useful in severely injured patients where intubation and ventilation has to be done immediately which limits clinical examination.
Which patient should undergo CT scan?
There are certain clinical decision rules which help us in deciding which patients require CT head. These rules help us in avoiding unnecessary radiation exposure without missing major injuries. It includes:
The Canadian CT Head Rule.
The New Orleans Criteria.
S100 protein estimation.
Canadian CT Head Rule: It is not applied to patients with GCS less than 13, open skull fracture, age less than 16 years, anticoagulated patients and non-trauma patients.
High risk for neurosurgical intervention
GCS<15 at 2 hours after injury.
Suspected open or depressed skull fracture.
Any sign of skull base fracture (haemotympanum, raccoon eyes, otorrhoea/ rhinorrhoea, battle sign).
2 or more episodes of vomiting.
Age more than or equal to 65 years.
Medium risk for brain injury
Amnesia before impact of more than or equal to 30 minutes.
Dangerous mechanism (ejected from a motor vehicle, struck by a motor vehicle, fall from a height of 3 feet or more or 5 stairs).
New Orleans Criteria: This is applied only when GCS is 15. CT is required for minor head injury patients with any one of the following
Headache.
Vomiting.
Age more than or equal to 60 years.
Drug or alcohol intoxication.
Persistent anterograde amnesia.
Visible trauma above the clavicle.
Seizure [28].
S100 Protien Estimation: More recently, estimation of serum levels of S100 protein in mild head injury patients has shown to help in deciding if the patient should undergo CT scan or not. In mild head injury patients, if serum S100 protein levels are less than 0.1 microgram/L, CT is not necessary. This helps in reducing unnecessary radiation exposure in mild head injury patients [29].
Magnetic Resonance Imaging (MRI) is the imaging modality of choice to identify Diffuse Axonal Injury (DAI), especially with diffusion weighted sequence. PET SCAN is helpful in assessing the cerebral blood flow in patients with traumatic head injury [30].
When to advise a facial CT along with CT Brain in patients with head injury?
There are certain facial lacerations which correlate with high chances of facial fractures underneath. These can be remembered with the mnemonic “LIPS-N (Lip laceration, intra oral laceration, periorbital contusion, sub conjunctival haemorrhage, nasal laceration) [31].
Types Of Head Injury: Head injuries include skull fractures and traumatic brain injuries. Traumatic brain injuries in turn can be focal or diffuse. Diffuse brain injuries include concussion and diffuse axonal injury. Focal brain injuries include cerebral contusions, subarachnoid haemorrhage, subdural haematoma and epidural haematoma.
Skull Fractures: Patients with cranial bone fractures with associated loss of consciousness have a high risk of intracranial haematomas [32]. The term “Bursting fracture” is used when there is a complex fracture with radiating fracture lines with comminuated fragments and associated soft tissue laceration. In young patients with soft and malleable bone, the so called “ping pong fractures” are seen. Here the bone becomes dented without any laceration of the skin and the dura [7]. Depressed skull fractures have a high chance of infection which is around 1.9-10.6%. A 90% of depressed fractures are open fractures [33]. Temporal bone fractures have a risk of sensorineural hearing loss or vestibular symptoms. An 80% of these are longitudinal and 50% involve facial nerve. Cranial bone fractures are often associated with Cerebrospinal fluid (CSF) leak [6].
CSF Leak: CSF leak can happen because of trauma to ethmoid and its cribriform plate, frontal sinus, anterior skull base and orbital roof. Most of the times the patient presents with features like rhinorrhoea, otorrhoea, headache, decreased hearing sensation and a salty taste. Clinically, it can be diagnosed with a double ring or a halo sign, where there is a larger and clearer CSF ring with a central small ring formed with blood. Laboratory tests such as glucose oxidase and β2 transferrin can be used. Since β2 transferrin is seen only in CSF, aqueous humour and perilymph, it can easily be used as a marker for detecting CSF leak. Various imaging modalities to look for CSF leak include CT, MRI, and cysternography. The best imaging modality available to locate the exact site of leak is high resolution CT with intrathecal metrizamide contrast medium. A 3-50% of the patients may end up with meningitis as a complication. An 85% recover spontaneously in 1 week’s time [34]. Conservative management include bed rest with head end elevation of 35 to 45 degrees. Patients must be advised not to do activities that can increase intracranial pressure such as blowing the nose, sneezing etc. If the leak is not resolved in 72 hours, a lumbar drain is placed. Even after this if the leak persists for more than 8 days, surgery is indicated. Either intracranial approach using craniotomy or extracranial endoscopic approach can be used for the same [35].
Concussion: It is defined as “alteration of consciousness without structural damage as a result of non-penetrating TBI” [36]. Alteration of consciousness can be in the form of confusion, loss of consciousness or amnesia. The hallmark of concussion is amnesia [36].
Extradural Haematoma (EDH): [Table/Fig-4] This occupies the extradural space between the skull and the dura. It occurs because of the rupture of middle meningeal artery which is most commonly associated with skull fracture [6]. EDH occurs mostly because of low velocity lateral blow to the temporal or the temporoparietal regions. Clinically most of the times, patient presents with coma because of the compression of brain due the bleed. An 80% of the times there may be a lucid interval and 60% of the patients may have dilated pupils, mostly on the ipsilateral side [37]. Seizures and contralateral hemiparesis can also occur.
Axial slice of computed tomogram illustrating Extradural Haematoma on the left side.
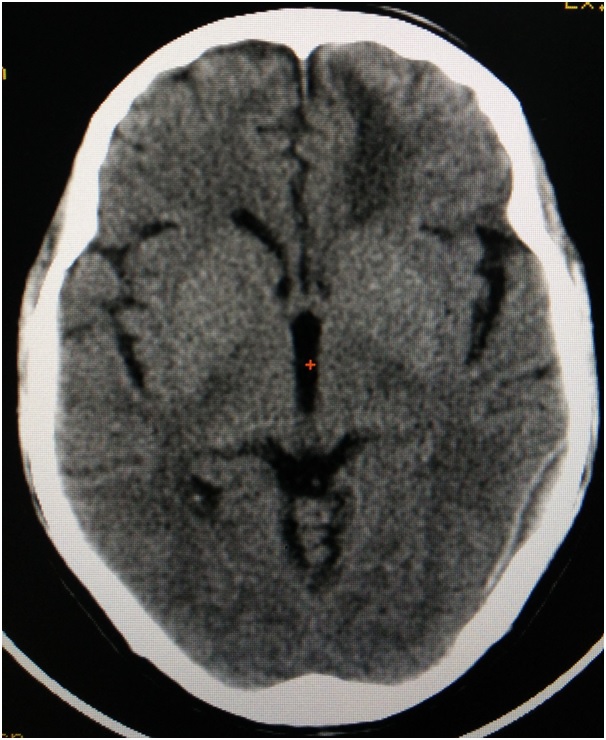
CT findings include biconvex hyperdense collection of blood. Pupil sign is used as an indicator for prognosis and patients with more than 150 ml of bleed will show poor prognosis. Surgery is advised to evacuate the haematoma within 4 hours of the injury [30]. Any volume of more than 30cm3, irrespective of GCS score is indicated for surgery [6].
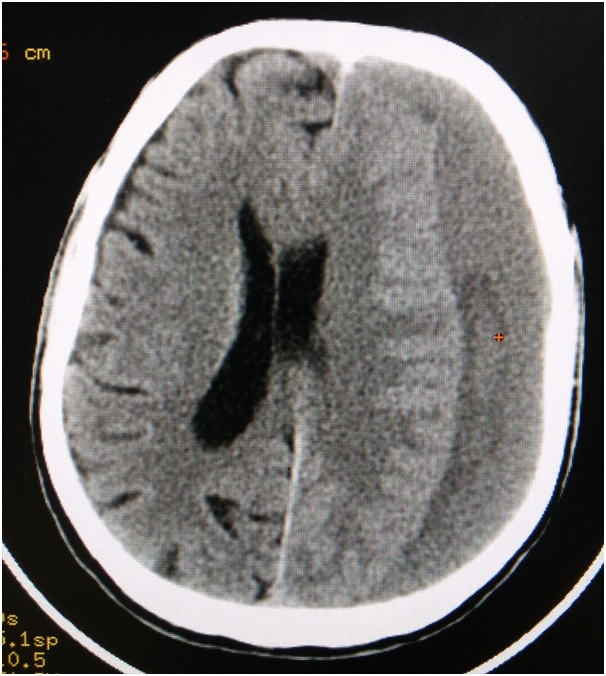
Subdural Haematoma (SDH): Around 30% of all TBI accounts to subdural haematoma. It can be acute, subacute or chronic based on the duration. Acute SDH are those that occur within 1-2 days of injury because of damage to the bridging veins, contusions, or penetrating brain injury. Subacute are usually 3-14-day-old. Any SDH older than 15 days are considered as chronic SDH [1]. It occurs due to the rupture of the cortical veins. Long duration causes the haemoglobin and other proteins to break down. This attracts fluid because of the increased osmotic pressure, resulting in the displacement of the brain, thus leading to coma or death. A 50% of patients have a history of trauma. SDH shows crescent shaped appearance in the CT. Chronic SDH appears as isodense or hypodense in CT [Table/Fig-5] and acute SDH appears hyperdense [Table/Fig-6]. Patients with low GCS and neurological deficits should undergo surgery. Surgical options include burr-hole drainage, mini craniectomy or twist drill craniostomy [30].
Axial slice of computed tomogram illustrating a chronic subdural haematoma on the left side.
Axial slice of computed tomogram illustrating an acute subdural hematoma on the left side.
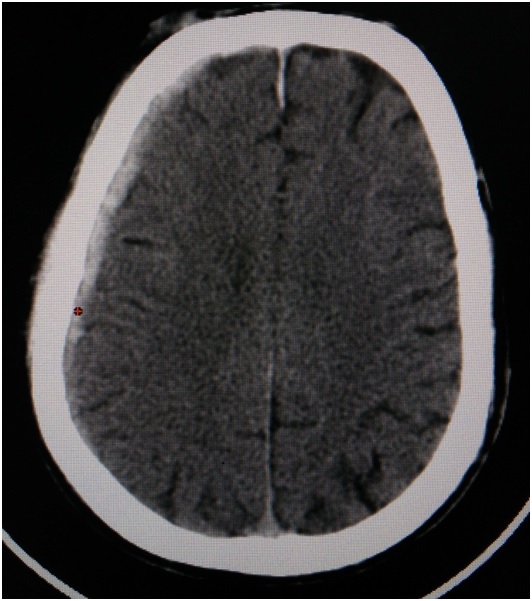
Diffuse Axonal Injury (DAI): DAI accounts for 40-50 % of all TBI. DAI can be suspected in any patient with loss of consciousness following trauma. It can occur at the junction of gray and white matter, corpus callosum and in the brainstem [38]. Acceleration or deceleration forces to brain can result in DAI. It is graded based on the location of petechial haemorrhages. There is also a deposition of β-amyloid precursor protein. MRI is the imaging modality of choice to identify DAI especially with diffusion weighted sequence. Magnetic Resonance spectroscopy can also be used [30].
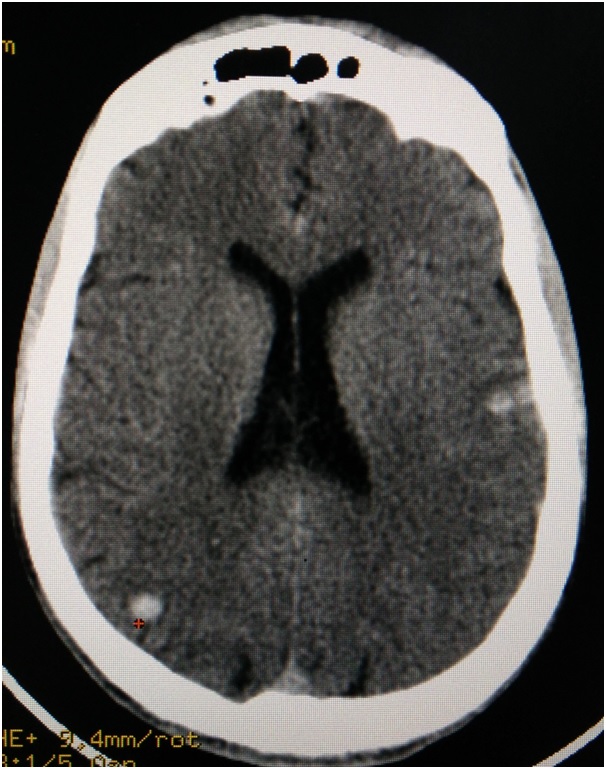
Cerebral Contusions: Contusions within the brain substance are frequently associated with acute subdural haematomas. It accounts for around 40% of the total TBI [39]. They may occur as a result of delayed bleeding. In CT, it appears as round hyper dense areas with some amount of oedema and a mild mass effect [Table/Fig-7]. It has to be operated if the ICP cannot be controlled or if there is worsening of the clinical conditions. Mortality associated with cerebral contusions is around 75% [40]. Prognosis is worse if it is associated with subdural haematomas, or if it is bilateral.
Axial slice of computed tomogram illustrating a cerebral contusions on the left and the right side.
Cerebral Perfusion Pressure (CPP): Knowledge about cerebral perfusion and autoregulatory mechanisms of brain is essential to prevent any secondary ischaemic damage. CPP is calculated by the difference between the mean arterial pressure (MAP) and the Intracranial pressure(ICP).
CPP=MAP-ICP
[41].
The autoregulatory mechanism in the brain can maintain the cerebral blood flow even when cerebral perfusion pressure ranges from 40 to 140 mmHg [42]. Normal cerebral perfusion pressure is 70-100 mmHg. Normal intra cranial pressure ranges from 7 to 15 mmHg [43]. ICP more than 20 mmHg is considered harmful [6]. Any condition which increases the ICP such as TBI can decrease the cerebral perfusion pressure leading to ischaemic damage secondary to TBI.
Loss of cerebral autoregulation results in cerebral oedema. Cerebral oedema can be of two types: Malignant and true. Malignant cerebral oedema results from rapid increase in the brain swelling and has 100% mortality rate, whereas true cerebral oedema occurs within few hours of head injury. To prevent cerebral oedema, we need to keep ICP under 20 mmHg and CPP above 50 mm Hg [44].
Intracranial Hypertension (IC-HTN): IC- HTN is seen in conditions like cerebral oedema, hyperaemia, traumatic haemorrhages, hydrocephalus, hypoventilation, systemic hypertension, venous sinus thrombosis and status epilepticus.
Clinical Signs of IC-HTN
1. Pupil dilatation (Unilateral and Bilateral).
2. Unequal response of pupils to light.
3. Progressive deterioration of neurological conditions which is not due to extracranial factors.
4. Decerebrate or decorticate posture.
ICP Monitoring: ICP should be monitored in severe TBI with GCS less than or equal to 8 with abnormal CT findings or patients with normal CT findings with 2 or more of the following risk factors such as age > 40, systolic BP < 90mmHg, decerebrate/decorticate posture. ICP is usually monitored using intra ventricular catheter or intraparenchymal monitor [36].
Management
• Intravenous (IV) Fluids: The choice of IV fluids in head injury is isotonic solution {Normal saline (NS) + 20 mEq potassium chloride (KCL)/L}. Intravenous volume has to be checked frequently to prevent increase in the ICP. Relatively hypotonic solution such as Ringers lactate should not be used as this will increase the ICP [36]. Care should be taken when administering glucose containing solutions as both hypo and hyperglycaemia can increase ischaemic damage to the brain [45].
• Prophylactic Antiepileptics: These can be used to prevent early post traumatic seizures (within 7 days of injury) but it cannot prevent late post traumatic seizures (after 7 days of injury). Phenytoin, carbamazepine and valproate are used for this purpose. Phenytoin should be administered with loading dose of 18mg/kg slow IV and maintenance dose of 200-500mg/day [36].
• ICP Management Protocol: The goal is to keep ICP less than 20 mm Hg and to keep CPP more than 50 mm Hg.
General Measures
• Positioning of the Patient: Head end elevation of 30 degrees will aid in the venous drainage and promote CSF diffusion into the spinal compartment. It decreases the mean carotid pressure and decreases ICP without affecting cerebral blood flow [46]. Head should be placed in the midline to prevent kinking of the jugular veins.
• Sedation and Neuromuscular Blockade: Lorazepam 1-2 mg IV q 4-6hourly or codeine 30-60 mg IM q 4 hourly can be used for light sedation [36]. This is used only when there is a clinical evidence of IC-HTN and when transportation is required. It should be kept in mind that it interferes with the neurologic examination and increases the chances of pneumonia. High dose barbiturates are given only if ICP cannot be controlled with other medical or surgical options [47]. Prophylactic use is not recommended.
• Monitor BP: Hypotension should be avoided. (Systolic BP should not be <90 mm Hg).
• Monitor Oxygenation: Avoid hypoxia (SpO2 <90%/ PaO2 <60mmHg).
• Prevent Hyperglycaemia: It is usually associated with head injury [48] and tends to increase cerebral oedema.
• Intubation: It is to be done if GCS is less than or equal to 8 or patients who continue to be hypoxic even with oxygen administration. Administration of antibiotics during intubation reduces the risk of pneumonia but mortality is unaffected [36].
• Prophylactic Hyperventilation: This should be avoided as it worsens cerebral ischaemia. It should be used only if IC-HTN is confirmed clinically or by CT. If required hyperventilate (PaCO2=30-35 mmHg and PaCO2 not less than 30 mmHg as it reduces cerebral blood flow). This should be avoided immediately after injury during first 24 hours [49].
• Prophylactic Hypothermia: It reduces the mortality if the target temperature of 32-35oC is maintained for more than 48 hours [50].
Specific Measures: These are undertaken in the following order, if the IC-HTN persists even after general measures-
1. Heavy sedation/ Paralytics should be given only after intubation [36].
2. Drain 3-5 ml of CSF which decreases the intracerebral volume using drip chamber with less than 10 cm from the external auditory canal [36].
3. Osmotherapy: These are the solutions which increase the osmotic pressure within the blood vessels thereby attracting the intra cranial fluid into the vascular compartment leading to decrease in the intracranial pressure. Osmotherapy is to be kept on hold if serum osmolarity > 320mOsm/L.
Mannitol: The dose is 0.25-1gm/kg bolus over <20 minutes followed by 0.25mg/kg over 20 minutes 6 hourly. Its prophylactic use is not recommended [36]. Mannitol is widely used to control ICP. Mannitol when used along with furosemide is more effective in reducing ICP. Recently hypertonic saline was found to be more efficient in decreasing ICP than mannitol [47].
Loop Diuretics (FUROSEMIDE): This can be used to alternate with mannitol at 10-20mg IV q 6 hourly. This acts by decreasing the ICP by decreasing cerebral oedema [36].
Hypertonic Saline: This is used in patients who are refractory to mannitol therapy to decrease the ICP. 3% saline of 25-50ml/hr IV can be used [36].
4. STEROIDS: CRASH (corticosteroid randomisation after severe head injury) study has shown that use of steroids in head injury patients had resulted in increased mortality rate within two weeks of injury. The reason for increased risk of death was unclear. Hence steroids are no more used in patients with head injury [51].
Conclusion
Many times, as maxillofacial surgeons, we encounter patients with maxillofacial trauma with concomitant head injury. Any patient with maxillofacial injury irrespective of whether it is associated with fractures or not, is always at a risk of traumatic brain injury. We should be able to suspect and diagnose head injury and also provide adequate initial management. Moreover all maxillofacial injury patients should then undergo neurosurgical observation and follow-up.
[1]. Krishnan DG, Systematic assessment of the patient with facial traumaOral Maxillofac Surg Clin N Am 2013 25(4):537-44. [Google Scholar]
[2]. Perry M, Advanced Trauma Life Support (ATLS) and facial trauma: can one size fit all? Part 1: dilemmas in the management of the multiply injured patient with coexisting facial injuriesInt J Oral Maxillofac Surg 2008 37(3):209-14. [Google Scholar]
[3]. Down KE, Boot DA, Gorman DF, Maxillofacial and associated injuries in severely traumatized patients: implications of a regional surveyInt J Oral Maxillofac Surg 1995 24(6):409-12. [Google Scholar]
[4]. Pappachan B, Alexander M, Correlating facial fractures and cranial injuriesJ Oral Maxillofac Surg 2006 64(7):1023-29. [Google Scholar]
[5]. Salentijn EG, Peerdeman SM, Boffano P, van den Bergh B, Forouzanfar T, A ten-year analysis of the traumatic maxillofacial and brain injury patient in Amsterdam: incidence and aetiologyJ Cranio-Maxillo-fac Surg 2014 42(6):705-10. [Google Scholar]
[6]. Neurologic evaluation and management. In: Fonseca RJ, Walker RV, Barber HD, Powers MP, Frost DE, editorsOral and maxillofacial trauma 2013 4th edMissouriElsevierIn. [Google Scholar]
[7]. Kiersch TA, Kaufman JB, Assessment of head injuriesJ Oral Surg Am Dent Assoc 1965 1974 32(4):269-74. [Google Scholar]
[8]. Keenan HT, Brundage SI, Thompson DC, Maier RV, Rivara FP, Does the face protect the brain? A case-control study of traumatic brain injury and facial fracturesArch Surg Chic Ill 1960 1999 134(1):14-17. [Google Scholar]
[9]. Haug RH, Savage JD, Likavec MJ, Conforti PJ, A review of 100 closed head injuries associated with facial fracturesJ Oral Maxillofac Surg 1992 50(3):218-22. [Google Scholar]
[10]. Kloss F, Laimer K, Hohlrieder M, Ulmer H, Hackl W, Benzer A, Traumatic intracranial haemorrhage in conscious patients with facial fractures–a review of 1959 casesJ Cranio-Maxillo-fac Surg 2008 36(7):372-77. [Google Scholar]
[11]. Hohlrieder M, Hinterhoelzl J, Ulmer H, Hackl W, Schmutzhard E, Gassner R, Maxillofacial fractures masking traumatic intracranial hemorrhagesInt J Oral Maxillofac Surg 2004 33(4):389-95. [Google Scholar]
[12]. Bouamra O, Wrotchford A, Hollis S, Vail A, Woodford M, Lecky F, Outcome prediction in traumaInjury 2006 37(12):1092-97. [Google Scholar]
[13]. Rajandram RK, Syed Omar SN, Rashdi MFN, Abdul Jabar MN, Maxillofacial injuries and traumatic brain injury–a pilot studyDent Traumatol 2014 30(2):128-32. [Google Scholar]
[14]. Advanced Trauma Life Support ATLS 9th Edition Student Course Manual | booksmedicos [Internet]. [cited 2015 Nov 1]. Available from: http://booksmedicos.org/advanced-trauma-life-support-atls-9th-edition-student-course-manual/ [Google Scholar]
[15]. Perry M, Morris C, Advanced trauma life support (ATLS) and facial trauma: can one size fit all? Part 2: ATLS, maxillofacial injuries and airway management dilemmasInt J Oral Maxillofac Surg 2008 37(4):309-20. [Google Scholar]
[16]. Kuhn JE, Graziano GP, Airway compromise as a result of retropharyngeal haematoma following cervical spine injuryJ Spinal Disord 1991 4(3):264-69. [Google Scholar]
[17]. Jung JY, Airway management of patients with traumatic brain injury/C-spine injuryKorean J Anaesthesiol 2015 68(3):213-19. [Google Scholar]
[18]. Chen JW, Cervical Spine InjuriesOral Maxillofac Surg Clin 2008 20(3):381-91. [Google Scholar]
[19]. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG, Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristicsJ Neurosurg 2002 96(3 Suppl):285-91. [Google Scholar]
[20]. Morris CG, Mullan B, Clearing the cervical spine after polytrauma: implementing unified management for unconscious victims in the intensive care unitAnaesthesia 2004 59(8):755-61. [Google Scholar]
[21]. Stiell IG, Clement CM, McKnight RD, Brison R, Schull MJ, Rowe BH, The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with traumaN Engl J Med 2003 349(26):2510-18. [Google Scholar]
[22]. Teasdale G, Jennett B, Assessment and prognosis of coma after head injuryActa Neurochir (Wien) 1976 34(1-4):45-55. [Google Scholar]
[23]. Udekwu P, Kromhout-Schiro S, Vaslef S, Baker C, Oller D, Glasgow Coma Scale score, mortality, and functional outcome in head-injured patientsJ Trauma 2004 56(5):1084-89. [Google Scholar]
[24]. Zuercher M, Ummenhofer W, Baltussen A, Walder B, The use of Glasgow Coma Scale in injury assessment: a critical reviewBrain Inj 2009 23(5):371-84. [Google Scholar]
[25]. Ross SE, Leipold C, Terregino C, O’Malley KF, Efficacy of the motor component of the Glasgow Coma Scale in trauma triageJ Trauma 1998 45(1):42-44. [Google Scholar]
[26]. Salottolo K, Levy AS, Slone DS, Mains CW, Bar-Or D, The effect of age on Glasgow Coma Scale score in patients with traumatic brain injuryJAMA Surg 2014 149(7):727-34. [Google Scholar]
[27]. Shackford SR, Wald SL, Ross SE, Cogbill TH, Hoyt DB, Morris JA, The clinical utility of computed tomographic scanning and neurologic examination in the management of patients with minor head injuriesJ Trauma 1992 33(3):385-94. [Google Scholar]
[28]. Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM, Indications for computed tomography in patients with minor head injuryN Engl J Med 2000 343(2):100-05. [Google Scholar]
[29]. Calcagnile O, Undén L, Undén J, Clinical validation of S100B use in management of mild head injuryBMC Emerg Med 2012 12:13 [Google Scholar]
[30]. Tsang KK-T, Whitfield PC, Traumatic brain injury: review of current management strategiesBr J Oral Maxillofac Surg 2012 50(4):298-308. [Google Scholar]
[31]. Holmgren EP, Dierks EJ, Assael LA, Bell RB, Potter BE, Facial soft tissue injuries as an aid to ordering a combination head and facial computed tomography in trauma patientsJ Oral Maxillofac Surg 2005 63(5):651-54. [Google Scholar]
[32]. Hung CC, Chiu WT, Lee LS, Lin LS, Shih CJ, Risk factors predicting surgically significant intracranial haematomas in patients with head injuriesJ Formos Med Assoc Taiwan Yi Zhi 1996 95(4):294-97. [Google Scholar]
[33]. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Surgical management of depressed cranial fracturesNeurosurgery 2006 58(3 Suppl):S56-60.discussion Si – iv [Google Scholar]
[34]. Mincy JE, Posttraumatic cerebrospinal fluid fistula of the frontal fossaJ Trauma 1966 6(5):618-22. [Google Scholar]
[35]. Brandt MT, Jenkins WS, Fattahi TT, Haug RH, Cerebrospinal fluid: implications in oral and maxillofacial surgeryJ Oral Maxillofac Surg 2002 60(9):1049-56. [Google Scholar]
[36]. Mark S Greenberg, Handbook of Neurosurgery 2010 7th edCanadaThieme [Google Scholar]
[37]. Larner AJ, A topographical anatomy of false-localising signsAdv Clin Neurosci Rehab 2005 5:20-21. [Google Scholar]
[38]. Le TH, Gean AD, Neuroimaging of traumatic brain injuryMt Sinai J Med N Y 2009 76(2):145-62. [Google Scholar]
[39]. Provenzale J, CT and MR imaging of acute cranial traumaEmerg Radiol 2007 14(1):1-12. [Google Scholar]
[40]. Cohen TI, Gudeman SK, Delayed traumatic intracranial haematoma. In: Narayan RK, Wilberger Jr JE, Povlishock JT. editorsNeurotrauma 1996 New YorkMcGraw-Hill:689-701. [Google Scholar]
[41]. Trivedi M, Coles JP, Blood pressure management in acute head injuryJ Intensive Care Med 2009 24(2):96-107. [Google Scholar]
[42]. Kelly BJ, Luce JM, Current concepts in cerebral protectionChest 1993 103(4):1246-54. [Google Scholar]
[43]. Smith M, Monitoring intracranial pressure in traumatic brain injuryAnaesth Analg 2008 106(1):240-48. [Google Scholar]
[44]. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholdsJ Neurotrauma 2007 24(Suppl 1):S55-58. [Google Scholar]
[45]. Conforti PJ, Haug RH, Likavec M, Management of closed head injury in the patient with maxillofacial traumaJ Oral Maxillofac Surg 1993 51(3):298-303. [Google Scholar]
[46]. Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patientsJ Neurosurg 1992 76(2):207-11. [Google Scholar]
[47]. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. XI. Anaesthetics, analgesics, and sedativesJ Neurotrauma 2007 24(Suppl 1):S71-76. [Google Scholar]
[48]. Bhattacharjee S, Layek A, Maitra S, Sen S, Pal S, Gozi NK, Perioperative glycemic status of adult traumatic brain injury patients undergoing craniotomy: a prospective observational studyJ Neurosurg Anaesthesiol 2014 26(4):313-19. [Google Scholar]
[49]. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. XIV. HyperventilationJ Neurotrauma 2007 24(Suppl 1):S87-90. [Google Scholar]
[50]. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. III. Prophylactic hypothermiaJ Neurotrauma 2007 24(Suppl 1):S21-25. [Google Scholar]
[51]. Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trialLancet Lond Engl 2004 364(9442):1321-28. [Google Scholar]