The World Health Organization has approximated that there are about more than 2 billion Hepatitis B virus infected people, about 378 million chronic carriers all over the world [1] and about 80 million HBV carriers in the Southeast Asia region [2]. HBV infection is considered as a cause of more than 50% of liver cancers. India is considered as intermediary endemic city of hepatitis B, with prevalence of hepatitis B surface antigen between 2% and 10% among the studied population [2].
Various screening and confirmatory tests have evolved for detection of hepatitis B status. Currently viral antigen and antibodies are detected by traditional serological tests. But the relative inconvenience of obtaining blood samples due to invasive nature of procedure and potential risks of disease transmission through needle stick injuries make serological testing unattractive [3]. However, the introduction of oral fluid as an alternative which is a non-invasive alternative to venipuncture or fingerprick has led to many researches. Various studies have emphasized the potential use of oral fluid as an alternative method to clinical diagnosis of infectious diseases and also a method to assess immunity levels of important vaccine-preventable virus infections [4]. This method keeps away from the phobia of venipuncture or fingerprick in addition, oral fluid samples can be self-collected by the patient. The use of oral fluid samples to identify antibodies to hepatitis viruses introduces a simple and alternative method to screen acute infection and immunity by means of body fluids that are easy to collect and will facilitate the investigation, the follow-up of the outbreak, and to monitor the candidates for vaccination against this disease [3]. Thus, the saliva offers enormous advantages over blood and is a valuable tool for screening and diagnostic purposes. Hence the present study was carried out to detect the presence of hepatitis antigen, its specificity and sensitivity in saliva by ELISA test.
Materials and Methods
The present prospective randomized study group comprised of 80 patients who visited Government Medical and Dental College and Hospital, Aurangabad, Maharashtra. An informed and written consent was obtained from individual patients. Ethical committee approval was also obtained. Inclusion criteria for sensitivity were hepatitis B positive patients with no other major illness. For specificity patients with no history of hepatitis B, healthy, well oriented OPD patients with no sign and symptoms of hepatitis B. Exclusion criteria for the study was patients with presence of salivary dysfunction leading to xerostomia or any major illness along with hepatitis B. The patient were divided into two groups, Group A comprised of 40 known hepatitis B seropositive patients and was considered for determining the sensitivity. Out of 40 patients 10 were of acute hepatitis, 28 were of chronic hepatitis and 2 were of hepatocellular carcinoma and the patients were on medication (either symptomatic or definitive). Group B comprised of 40 known hepatitis B seronegative patients and considered for determining the specificity. All the 40 seropositive patients were taken from isolated ward of medical college and hospital and a detailed medical, personal and family history was documented for all the cases. The seronegative patients selected for the study were OPD patients with no history, sign and symptoms related to hepatitis. The selected subjects volunteered to undergo blood and oral sample testing for HBsAg. A 2-3 ml unstimulated oral fluid samples were collected from both the groups at convenience in a wide mouthed ice cooled test tube. During oral fluid collection, any sample containing visible blood from the inflamed gingival tissues was discarded. And under all aseptic precautions 1.5-2 ml of venous blood was drawn from the ante-cubital fossa using a 24 guage disposable needle and 5ml syringe and kept aside for 1 hr to coagulate and serum to be separated. Before storing the samples, saliva (oral fluid) samples were incubated for 16 hours and then centrifuged for 15 minutes and the supernatant was collected in a sterile test tube with cap and labeled. Similarly serum was separated from the coagulated blood and also centrifuged for 15 minutes and labeled. Both the samples were then stored at -20oc until analysis. Before commencing with the procedure, samples were thawed to room temperature. HBsAg was evaluated in the serum and saliva of the subjects from both group A and B using Diasorin’s ETI-MAK-4 ELISA KIT. The method utilizes multiple-well microtiter plates, coated with capture antibodies, to capture soluble proteins. The bound proteins are then detected with a subsequent detection antibody, which is typically labeled with an enzyme, or biotinylated and then followed with streptavidin-enzyme conjugate. A colorimetric substrate is then added, which results in a color change based on the amount of antigen captured. By using a plate reader and plotting resulting values on a standard curve, precise, quantitative values can be obtained [5]. The procedure was standardized for detection of the antigen in oral fluid as instructed by the manufacturer with positive and negative controls provided in the kit. On completion of the method, the readings (absorbance values) were obtained from an ELISA reader adjusted to the wavelength of 450nm. The A450 values obtained on the serum samples were than compared with the A450 values of the corresponding oral fluid samples. Same steps were followed for oral fluid and serum except for slight modification in the primary incubation period of oral fluid which was 16 hours.
Sensitivity and specificity was calculated as:
Sensitivity = A/A+C × 100
= 40/40+0× 100
= 100 %
where A is true positive patients and C is false positive patients.
Specificity = D/D+B×100
= 40/40+0×100
= 100 %
where B is false positive patients and D is true negative patients.
Mann-Whitney U test was used to test the statistical significance between serum and oral fluid samples p-value <0.05 was considered as significant value. The Kappa(K) statistic was used to assess the degree of agreement between oral fluid and serum.
Results
With a cut-off value 0.069, signal to cutoff ratio (absorbance value divided by cut-off value) was obtained and S/CO> or=1.1 considered as a positive result; S/CO< or=0.9 as a negative test results (according to manufacturer’s guidelines) reliability of oral fluid for diagnosis of hepatitis B was measured by calculating sensitivity and specificity. Out of the 40 seropositive cases of hepatitis B infection all 40 were positive with oral fluid and serum samples. On the other hand all the 40 seronegative cases of hepatitis B infection were negative with oral fluid and serum samples too. The calculations of the data revealed 100% sensitivity and specificity of oral fluid. Mann-Whitney U test was used to test the statistical significance between serum and oral fluid samples. The mean score of serum absorbance value was found to be 1.8084+0.4223 and for saliva was 0.7729+0.2602 [Table/Fig-1] in seropositive patients. p-value <0.0001 obtained and found extremely significant [Table/Fig-2,3]. Result of kappa statistic tabulated in [Table/Fig-4]. Kappa value (K) =1 suggests perfect agreement between oral fluid and serum for diagnosis of Hepatitis B. (K=1 indicates perfect agreement, K=0 indicates no better than that expected by chance and K<0 worse than that by chance).
Comparison of mean absorbance values between serum and saliva in seropositive cases.
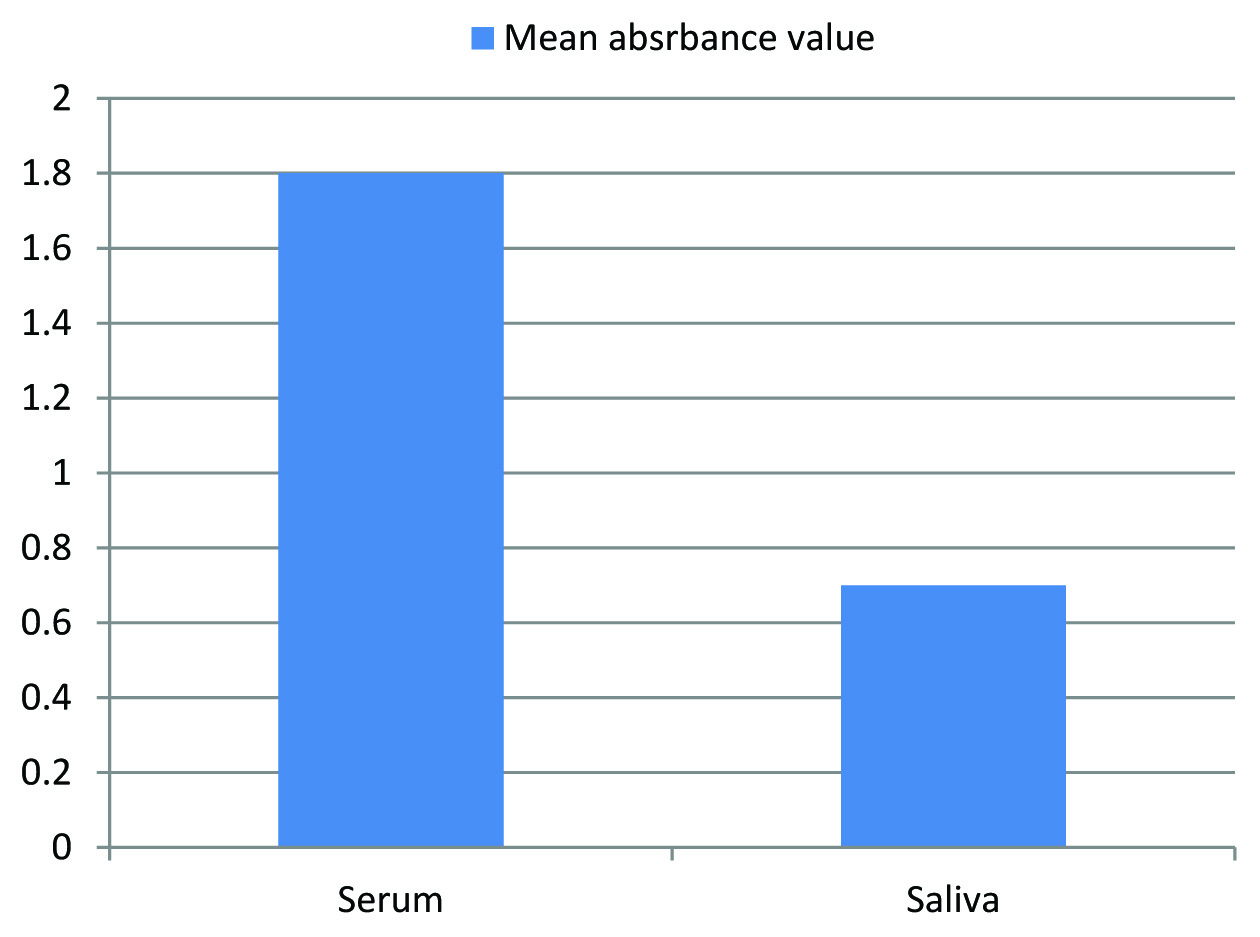
Mean values serum and oral fluid samples.
| Medium | No. of samples | Mean±SD | p-value (Mann-Whitney U test) |
|---|
| Serum | 40 | 1.8084±0.4223 | p<0.0001 |
| Oral fluid (Saliva) | 40 | 0.7729±0.2602 | |
Comparison of serum and saliva samples using ELISA kit.
Result of kappa statistic used to assess the degree of agreement between oral fluid and serum.
| SALIVA | SERUM | Observed frequency | Agreement by chance | Kappa value | Status of agreement |
|---|
| Results | Reactive | Non-reactive | Total |
|---|
| Reactive | 40 | 0 | 40 | 80 | 40 | 1 | perfect |
| Non-reactive | 0 | 40 | 40 |
| Total | 40 | 40 | |
Discussion
Hepatitis B is a global health issue. Hepatitis B virus (HBV) is regarded as second only to tobacco as a major cause of cancer [6]. Although most developed countries are showing good trend towards decline of HBV, developing countries including India have shown no evidence of decline [7].
Hepatitis B virus is present in all the bodily secretions i.e. blood, sweat, saliva, tears, nasopharyngeal fluid, menstrual blood, vaginal secretions, semen, breast milk and urine of infected persons [8]. Mode of transmission of HBV is vertical, parenteral or sexual contact with infected individual [9]. Recently, Hui AY et al., reported a case of transmission of hepatitis B via saliva [10]. Acute hepatitis B was developed after human bite by chronic HBV carrier. The case of transmission was confirmed after detection HBV DNA in the saliva of biter and analysis revealed that genotype and sequence of HBV present in both subjects were identical.
Thus, however HBV is present in wide varieties of bodily fluids, blood is the only fluid regularly used in tests of viral antigens and antibodies. But, as serum is inconvenient to collect, possess potential risks of disease transmission through needle stick injury and problems are compounded if the persons to be tested are children, intravenous drug users and obese persons [3]. Oral fluid as an alternative to blood could provide substantial advantages, since it is less invasive, less painful, less expensive, no trained personal required, safe, and large samples can be collected for epidemiological and prevalence study [11].
Krastava A et al., collected serum and saliva samples from patients with positive sera and detected HBV DNA levels varying from 494 to 6300000000 cp/ml [12]. HBV DNA levels in saliva and serum were quite similar in cases with serum HBV DNA < 10 000 cp/ml. Peginterferon α-2a treatment was initiated in five patients to further evaluate the serum and salivary response to levels of HBV DNA and it was revealed that subjects remained viraemic during treatment period with persistently detectable HBV DNA levels in saliva, too. Hence, demonstrated the role of saliva in routes of HBV transmission. Thus, the present study was planned and conducted to assess the saliva which is an easily available biological fluid for detection of HBsAg which is the hallmark of infection. So, our aim was to assess the positivity of salivary HBsAg with that of serum HBsAg and to test the reliability of saliva as a diagnostic tool. HBsAg can be detected in the serum from several weeks before onset of symptoms to months after onset. HBsAg is present in serum during acute infections and persists in chronic infections. The presence of HBsAg indicates that the person is potentially infectious [13]. In the present study oral fluid was incubated for 16 hours. Similar modification was also recommended by Hutse V et al., [11]. Cruz HM et al., also revealed that the highest sensitivity and specificity could be obtained by increasing the incubation of sample and conjugation to 16 hour and by the use of the area under the receiver operating characteristic curve to calculate cut-off values [14]. The samples were stored at -20 0C until analysis. Analysis was carried out after the collection of all the samples. Both HBV antigen and antibody are stable at room temperature for days, at 40C for months and -200C to -700C for many years [15]. In the present study out of total 80 patients 40 seropositive and 40 seronegative, HBsAg was found in the oral fluid of all seropositive patients and none of the seronegative patients had found HBsAg, indicating specificity and sensitivity of 100%. Our results are in concurrence with the study carried out by Thieme T et al., in which sensitivity and specificity of oral sampling compared with serum sampling were 100% for hepatitis B virus surface antigen [16]. Similar results were also found by Piacentini SC et al., and George JR et al., [17,18]. However, in previously conducted studies, variation in the percentage of detection from the 30-100% have been observed by authors which may be due to variation in mode of collection, handling of oral fluid and technique used for analysis of HBsAg as well as population studied. The studies conducted earlier were among western population. Banvar et al., carried out a study among Indian population and found sensitivity of 45% and specificity of 100% of oral fluid [4]. In the present study, the mean score of serum was 1.8084 and that of oral fluid 0.7729 and obtained p-value was <0.0001 in seropositive patients, suggesting that the antigen concentration in serum is comparatively higher than the oral fluid. Degree of agreement between oral fluid and serum antigen status was 1 which imply perfect agreement between oral fluid and serum antigen for detection of hepatitis B infection. Amado LA et al., also found almost perfect agreement, the kappa coefficient of 100% indicated an excellent agreement of serum and saliva results, suggesting that this marker can be used as a diagnostic tool for recent HBV infection in oral fluid [3]. Cruz HM et al., also found excellent agreement between the results for the saliva and serum specimens, kappa value κ: 0.87 for whole saliva and γ: 0.80 for oral fluid [14]. Nokes DJ et al., also stated fair agreement between oral fluid and serum antigen status and suggested that oral fluid has the potential to replace serum to evaluate population immunity levels [19].
Conclusion
The convenience, reliability and non invasive nature of oral fluid make it an attractive alternative to serum for hepatitis B detection and taking into the account of sensitivity and specificity of this oral fluid test, it is seen that oral fluid can be used for detection of HBsAg. It can be utilized for large scale hepatitis B viral screening in rural areas where blood collection, serum separation and storage might be difficult or economically unfavourable. Therefore concluding, oral fluid testing can be an interesting alternative for HBV screening and epidemiological studies. However, we cannot exclude need for further research on large sample size before implementing oral fluid as a diagnostic tool.