Open-label, Prospective, Investigator Initiated Study to Assess the Clinical Role of Oral Natural or Synthetic Progesterone During Stimulated IUI Cycles for Unexplained Infertility
Jaideep Malhotra1, Korukonda Krishnaprasad2
1 Director, Global Rainbow Healthcare, Rainbow Hospitals, Agra, India.
2 Consultant Gynaecologist, Chembur, Mumbai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Korukonda Krishnaprasad, Gurudev Apartments, RC Marg, Chembur Naka, Mumbai-400071, India. E-mail : kkrishnaprasad@rocketmail.com
Background
Unexplained infertility remains as one of the important subtype of infertility that follows expectant management with Intrauterine Insemination (IUI) in most cases.
Aim
To evaluate the clinical role of progesterone supplement as luteal phase support for women with unexplained infertility following stimulation protocol with Clomiphene Citrate (CC)/Human Menopausal Gonadotropin (HMG).
Materials and Methods
An investigator initiated study to survey the success rate for first cycle of IUI following stimulation protocol with CC/HMG & luteal phase support with oral natural or synthetic progesterone was conducted. 120 patient records between observation period of Jan to May ’14 were retrieved especially for subjects undergoing IUI procedure for Unexplained infertility. Patients with baseline Serum (Sr). progesterone records who received Oral Natural Micronized Progesterone Sustained Release (Oral NMP SR) (N=45) or Dydrogesterone (n=33) following CC/HMG induction protocol and human Chorionic Gonadotropin(HCG) Inj., were further analysed following Luteal Phase Support(LPS) with oral natural or synthetic progesterone.
Results
Baseline demographics showed 78 patients with mean age, weight and cycle duration of 29.5 yrs, 57.3 kg & 28.6 days respectively. Progesterone was supplemented as Oral NMP SR 200/300 mg OD or Dydrogesterone 10 mg bid in 22, 23 and 33 patients respectively. In all cases ovulation was triggered with HCG inj., followed by IUI within the next 48 hours while baseline sr. progesterone levels were being assessed. Medicines and Healthcare Products Regulatory Agency (MHRA) UK recommended therapeutic compliance to suggest sr. progesterone levels of ≥14ng/ml were recorded as Mid-luteal levels in all of these patients. This therapeutic compliance was noted in 82.2% & 78.8% of the patients treated with oral NMP SR or Dydrogesterone respectively. Pregnancy was observed amongst 5 and 10 patients treated with oral NMP SR and Dydrogesterone respectively at the end of ‘First’ IUI cycle. Both the groups were well tolerated with drowsiness documented in three cases for Oral NMP SR.
Conclusion
Clinical supplementation with ONMPSR suggests therapeutic compliance and alternative strategy to conventional formulations while offering dosing convenience with minimal side effects.
Dydrogesterone, Luteal phase support, Oral NMP SR
Introduction
World Health Organization explains primary infertility as inefficiency to conceive after a year of unprotected sex and secondary if not conceived following previous pregnancy. According to WHO, the prevalence of primary infertility in India is between 3.9% to 16.8% [1]. National Family Health Survey (NFHS)-II estimates that 3.8% of women between the ages of 40 and 44 years have not had any children [2]. Significant risk factors include higher education, age at marriage >25, postponement of child bearing for ≥ 1 year, obesity, polycystic ovarian syndrome, irregular menstrual pattern, endometriosis, Sexually Transmitted Infections and age at menarche >14 years [3]. Amongst the various forms of infertility, unexplained infertility remains a common subtype with prevalence rate of about 10% [1]. Unexplained infertility, sometimes also called idiopathic infertility, refers to failure to conceive in a couple for whom no definitive cause for infertility can be found [4]. Common causes are Pituitary and or follicular dysfunction, gamete dysfunction and alterations in endometrial function [4]. Another possible explanation is an impaired luteal phase (demonstrated in about 30% of women with unexplained infertility), with a shorter luteal phase or a decreased peak serum progesterone level [4]. Intrauterine insemination (IUI) is often used for the management of unexplained infertility. Subsequent to clomiphene citrate/HMG induction stimulation protocol, HCG trigger is given followed by IUI and progesterone supplementation during the luteal phase to facilitate better implantation of the embryo followed by sustenance of pregnancy. The clinical utility of progesterone supplementation has been again highlighted by Frishman et al., with the endometrium showing ‘In phase’ changes with ‘Oral’ administration of 200 mg formulation thrice a day [5]. In this background, oral administration of natural micronized progesterone as sustained release (SR) preparation offers slow yet consistent systemic concentrations in the therapeutic range of ≥ 14 ng/ml (MHRA, 2008) for sustaining pregnancy while offering once a day dosage convenience for better patient compliance [6]. The slow, sustained release kinetics of progesterone by SR formulation minimizes immediate drug loading or exposure for any dose related side effects including drowsiness due to the active metabolite Allopregnanolone [6]. Oral NMP SR used worldwide since 1986 has been recently introduced in India for management of Luteal Phase Defect (LPD) and as Luteal Phase Support (LPS) during Assisted Reproductive Techniques (ART). Critical factors responsible for success of IUI include age, history of reproductive milestones, type of Controlled Ovarian Hyperstimulation (COH) or stimulation protocol used without underplaying the benefits of luteal phase support with progesterone supplementation.
Therefore the aim of the present study was to evaluate the clinical benefits and therapeutic compliance of progesterone supplements as LPS, an investigator initiated study was conducted at 5 centres across India. 1) Prachee Clinic and Research, Plot no. 1484, Sector 6, Cuttack – 753014; 2) SURGY centre, 149/4, Biren Roy Road (West), Kolkata-700061, West Bengal; 3) Malhotra Nursing and Maternity Home (P) Ltd, 84, Mahatma Gandhi Road, Agra- 10; 4) Akash Fertility Centre & Hospital 10, No 10, Jawaharlal Nehari Road (100 Ft Road), Vadapalani, Opposite Ambica Empire Hotel, Vadapalani, Chennai, Tamil Nadu 600026, 5) Global Rainbow Healthcare, Rainbow Hospitals, Agra to further assess the success rate for first cycle of IUI procedure in Unexplained infertility cases following stimulation protocol with CC/HMG and luteal phase support with oral natural or synthetic progesterone.
Materials and Methods
This Open label, prospective, Investigator Initiated observational surveillance study conducted between observation period of January to May 2014 in five out-patient settings across India with approval from an Independent Ethics Committee. The study was conducted as per International Conference for Harmonisation -Good Clinical Practise (ICH GCP) Guidelines, Schedule Y of Drugs and Cosmetic Act with written informed consent from patients.
The study population consisted of females aged 22 to 40 years with at least 1 year history of unexplained infertility. A total of 120 cases of unexplained infertility were evaluated in the study. Exclusion criteria included women with history of Progesterone (Natural or synthetic) use in the last cycle, history of tubal insufficiency or obstruction including Endometriosis or Polycystic Ovarian Syndrome, AST and/or ALT > 2.5 x ULN (active liver disease), Serum creatinine >1.5 mg/dl (active renal disease), Uncontrolled hypertension, hypercholesterolemia or diabetes, oral anticoagulants or prolonged use of high doses of NSAIDs, Hypersensitivity to Natural micronized progesterone, Spouse with Male factor infertility.
At baseline visit, detailed history, physical examination and medications of the subjects were recorded. Patients with baseline FSH <10mIU/ml or E (2) levels <80 pg/ml on Day 0+/-2 day of cycle with sr. progesterone levels were assessed on the day of IUI procedure respectively were included for the analyses.
In the next cycle, ovalogens including Clomiphene citrate (100 to 150 mg) was administered from 2nd to 6th day of cycle for 5 days. Each of the cycles was further stimulated with HMG for 3 to 5 days from Day 4 with regular monitoring of mono- or bi-follicular growth by ultrasound. The injections were continued till the ovarian follicles were large enough (≈16 to 18 mm) for HCG 5000-10000 IU trigger on 12th or 13th day to release the eggs. IUI procedure was conducted within the next 48 hours while assessing the baseline sr. progesterone levels. On the same day of HCG trigger, patients were prescribed Progesterone supplement i.e oral NMP SR 200/300mg OD or Dydrogesterone 10mg BD for 2 weeks. Serum progesterone levels were repeated on Day 21+/-2 of the stimulated cycles with pregnancy confirmation by biochemical and urinary estimation of beta-HCG subsequently after the ‘First’ IUI cycle.
In case of pregnancy being confirmed, the progesterone regimen was continued for upto 8 to 12 weeks further. Otherwise the drug regimen was discontinued after 2 weeks and appropriate therapy as justified by the doctor was followed.
Any side effects or serious adverse events including death, life-threatening (real risk of dying), hospitalization (initial or prolonged), disability (significant, persistent or permanent), congenital anomaly, required intervention to prevent permanent impairment or damage arising during the course of the study were documented and notified to Drug Controller General of India (DCGI) on the Suspected Adverse Drug Reaction reporting form of Central Drug Standard Control Organization (CDSCO). A post-hoc statistical analysis involving descriptive statistics and paired-t test utilizing IBM SPSS statistics v.21 software package was carried out to assess the level of significance with p< 0.05 considered as significant.
Results
Data records from 120 patients who underwent IUI procedure for unexplained infertility between the observation period was collated for analyses. Twelve of these patients had confounding Male factor subfertility or intermediate infertility amongst their partners and excluded from analyses. Amongst the rest, baseline sr. progesterone assessment records were available in 78 patients as highlighted in [Table/Fig-1].
Patient disposition chart at the end of the study.
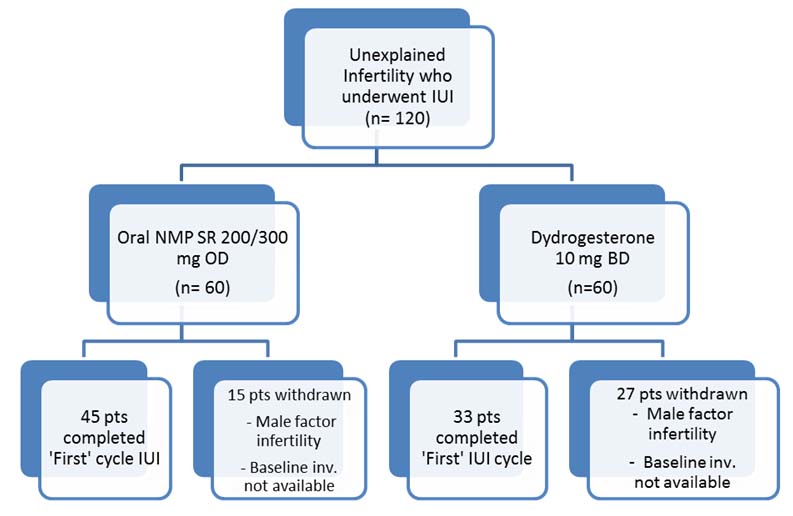
Of the 42 cases, 12 couples did not fulfill the exclusion criteria for Male factor infertility as suggested by WHO guidelines (2010) since the objective data on sperm motility (progressive or total) was missing. In the rest of the thirty (30) cases, baseline sr. progesterone levels were not available for analyses and therefore excluded from the Per Protocol analyses.
Baseline demographics showed mean age, weight and cycle duration distribution of 29.5 years, 57.3 kg & 28.6 days respectively. Progesterone was supplemented as Oral NMP SR 200/300 mg OD or Dydrogesterone 10 mg bid in 22, 23 and 33 patients respectively. In all cases ovulation was triggered with HCG Injection followed by IUI within the next 48 hours while baseline sr. progesterone levels were being assessed. The baseline demographics are presented in [Table/Fig-2].
Baseline demographics for patients undergoing IUI for Unexplained infertility.
| Oral NMP SR | Dydrogesterone |
|---|
| N | 45 | 33 |
| Age | 29 yrs | 30.1 yrs |
| Weight | 55.7 | 59.5 |
| Menses Duration | 29 days | 28.2 days |
| Obstetric History |
| G | 0.08 | 0.27 |
| P | 0.13 | 0.09 |
| A | 0.2 | 0.39 |
| L | 0.08 | 0.06 |
| Risk Factors | n | % | N | % |
| • Menstural irregularity | 5 | 11.11 | 4 | 12.12 |
| Medical History | n | % | N | % |
| • Type 2 Diabetes | 1 | 2.22 | 1 | 3.03 |
| • Hypothyroidism | 4 | 8.89 | 2 | 6.06 |
Ovulation Induction was done with Clomiphene citrate 100 mg for 5 days in 95% of patients and Inj HMG 75/150 mg for 3days in 98% of patients. Luteal phase support given with Dydrogesterone 10 mg BD in 33 patients and oral NMP SR 200mg OD in 22 patients and Oral NMPSR 300mg OD in 23 patients respectively.
The obstetric history was not significant with Secondary infertility noted in 13 (0.4%) and 7 (0.2%) patients for Dydrogesterone and oral NMP SR groups respectively. Amongst the cases of secondary infertility in oral NMP SR, 10% cases had history of stillbirth.
Mid-luteal sr. progesterone levels assessed in both groups on Day 21+/-2 showed mean levels of 30.7 and 28.5 ng/ml in both the groups for Dydrogesterone and oral NMP SR group respectively [Table/Fig-3].
Serum Progesterone levels in Dydrogesterone and Oral NMP SR groups. Pregnancy was observed amongst 5 (11%) and 10 (30%) patients treated with oral NMP SR and Dydrogesterone respectively during the ‘First’ cycle of IUI.
| Serum Progesterone | % change, p-value | % pts achievingmid-luteal sr. proglevel (≥14 ng/ml) |
|---|
| Baseline | Mid luteal |
|---|
| Dydrogesterone(10mg BD) | 4.2 | 30.7 | 86.3%, p=0.001 | 78.8% |
| OMP SR |
| (200mg OD) | 0.28 | 20.6 | 98.6%, p=0.000 | 82.2% |
| (300mg OD) | 2.02 | 36.1 | 94.4%, p=0.000 |
Adverse events: Oral NMP SR and Dydrogesterone were both found to be safe and well tolerated. Only 3 cases of drowsiness and 1 case of nausea were seen with OMP SR while 4 cases of nausea and 1 case of drowsiness reported with Dydrogesterone.
Discussion
Unexplained infertility continues to be an important cause of infertility with few treatment options available at the disposal of the clinician. Despite the varied aetiology involving immunologic, endocrinological or genetic reasons, the over-reliance on the use of ART techniques has never been underemphasized. In this line the clinical benefits and utility of Controlled ovarian Hyperstimulation plus intrauterine insemination as strategy has often been shown to be more effective than intrauterine insemination especially in terms of fecundity or pregnancy rates.
Success rate for patients undergoing IUI has been found to be varied. IUI in the spontaneous cycle among women with unexplained infertility yields pregnancy rates of 2.4-7.4% per cycle [7,8] which is similar to pregnancy rates of expectant management [8]. If CC is used without IUI, the pregnancy rate per cycle is 4-5.6% among women with unexplained infertility [7,9]. The use of gonadotropins (hMG) alone is associated with a pregnancy rate of 7.7% [7]. If CC is used in combination with IUI, the pregnancy rate per cycle is approximately 8.3% [7]. However, in case of COH + IUI, the pregnancy rate per cycle varies from 3.8 to 14.3% [10–13]. Though several factors could explain the divergent success rates reported for First cycle IUI procedure following Controlled Ovarian Hyperstimulation (COH) with CC/HMG stimulation protocol, LPS with progesterone has often been recommended for better ‘implantation’ and therefore successful ‘ongoing’ pregnancy. In this line, MHRA has recommended mid-luteal sr. progesterone levels as ≥14 ng/ml to adequately ‘sustain’ pregnancy especially in documented cases of luteal phase deficiency
A recently concluded open-label observational study conducted by Gopinath [6], to determine the success of first cycle IUI that was unstimulated or CC regulated involving LPS with oral natural or synthetic progesterone showed promising results. 93.3% of the patients in both the groups achieved ‘therapeutic’ mid-luteal sr. progesterone levels especially with oral NMP SR 400 mg group. Similarly pregnancy rate of 3.3 & 6.6% were noted in with Dydrogesterone and Oral NMP SR group respectively [6]. While in the present study, 82.2% of the patients achieved achieved mid luteal Serum. Progesterone levels ≥ 14 ng/ml with pregnancy rate of 11% (n=5) for Oral NMP SR group receiving 200 or 300 mg once a day for the ‘First’ cycle of Controlled Ovarian Hyperstimulation (COH) + IUI.
These results with oral NMP SR are similar to those reported by Jang et al., for women with unexplained infertility [14]. Jang reported pregnancy rates of 7.8% and 12% for women undergoing Natural or COH+IUI cycle compared with 6.6% and 11% reported by Gopinath et al., and current study respectively [6]. This open label, observational study further compliment the findings by Gopinath et al., regarding the clinical role of oral NMP SR in women with iatrogenic progesterone insufficiency due to CC/HMG cycles unlike the natural cycles that was earlier reported [6]. These clinical findings for the first cycle however need to be further confirmed in a reproducible, double blind, randomized clinical trial setting.
Conclusion
Clinical supplementation with oral NMP SR suggests therapeutic compliance and alternative strategy to conventional formulations while offering dosing convenience with minimal side effects.
[1]. Infecundity, infertility, and childlessness in developing countries. DHS Comparative Reports No 9; 2004. World Health Organization. Available from: http://who.int/reproductivehealth/publications/infertility/DHS_9/en. Accessed on 27th Nov 2015 [Google Scholar]
[2]. National Family Health survey (NHFS-3); 2005-2006 India. Available from: http://catalog.ihsn.org/index.php/catalog%20/2549. Accessed on 27th Nov 2015 [Google Scholar]
[3]. Saoji A, Primary infertility problems among female have been a source of concern in India LatelyInt J Med Res Health Sci 2014 4(1):332-40. [Google Scholar]
[4]. Isaksson R. Unexplained Infertility: Studies on aetiology, treatment options and obstetric outcome. Department of Obstetrics and Gynaecology, Helsinki University Central Hospital, Helsinki2002:13;15-16 [Google Scholar]
[5]. Frishman G, Klock S, Luciano A, Nulsen J, Efficacy of oral micronized progesterone in the treatment of luteal phase defectsJ Reprod Med 1995 40(7):521-24. [Google Scholar]
[6]. Gopinath P, Desai R, Open-label observational study to determine the success rate of first cycle Intra Uterine Insemination (IUI) involving luteal phase support with oral Natural or Synthetic progesteroneInt J Med Res Health Sci 2014 3(4):933-36. [Google Scholar]
[7]. Guzic D, Sullivan M, Adamson G, Cedars M, Falk R, Peterson E, Steinkampf M, Efficacy of treatment for unexplained infertilityFertil Steril 1998 70:207-13. [Google Scholar]
[8]. Goverde AJ, McDonnell J, Vermeiden JPW, Schats R, Rutten FFH, Schoemaker J, Intrauterine insemination or in-vitro fertilisation in idiopatic subfertility and male subfertility: a randomised trial and cost effectiveness analysisLancet 2000 355:13-18. [Google Scholar]
[9]. Hughes E, Collins J, Vandekerckhove P, Clomiphene citrate for unexplained subfertility in WomenCochrane database Syst Rev 2010 (1):CD000057 [Google Scholar]
[10]. Cohlen BJ, te Velde ER, Habbema JDF, Postcoital test should be performed as routine infertility testBr Med J 1998 318:1008 [Google Scholar]
[11]. Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JPW, Schoemaker J, Intrauterine insemination does and clomiphene citrate does not improve fecundity in couples with infertility due to male or idiopathic factors: a prospective, randomized, controlled studyFertil Steril 1990 53:847-53. [Google Scholar]
[12]. Nulsen JC, Walsh S, Dumez S, Metzger DA, A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertilityObstet Gynecol 1993 82:780 [Google Scholar]
[13]. Arici A, Byrd W, Bradshaw K, Evaluation of clomiphene citrate and human chorionic gonadotropin treatment: a prospective, randomized, crossover study during intrauterine insemination cyclesFertil Steril 1994 61:314 [Google Scholar]
[14]. Jang I, Hwang N, Analysis on pregnancy rate of Intra-Uterine Insemination in Unexplained Infertility in KoreaKorean J Reprod Med 2010 Available from: http://iussp.org/en/event/17/programme/paper/5506. Accessed on 2nd Nov 2015 [Google Scholar]