In the modern era of multidetector computed tomography (MDCT), it is advisable to revisit the anatomical variation of the celiac artery, the common hepatic artery and the hepatic arteries. Being aware of the normal anatomy and its variants is essential if iatrogenic injury to these structures is to be avoided. The abdominal aorta supplies the gut through three branches namely, the celiac axis, the superior mesenteric artery and the inferior mesenteric artery. Anomalies during embryogenesis however might result in a variety of anatomic variants [1].
The celiac artery originates from the abdominal aorta at the level of the twelfth thoracic vertebrae. It gives rise in turn to the left gastric artery (LGA), splenic artery and the common hepatic artery (CHA) [2].
The CHA is an arterial segment coursing from the celiac axis to the point where the gastroduodenal (GDA) artery arises, beyond which it becomes the proper hepatic artery. However, the CHA can have variant origin from other than the celiac axis and can have a different anatomic course. Song et al., redefined the CHA as an arterial segment containing at least one segmental hepatic artery and the gastroduodenal artery, irrespective of its origin and anatomic course [3]. In our study we use the above definition for the CHA. With recent increase in the frequency of the hepato-pancreatobiliary surgery, laproscopic surgery and liver transplantation, being aware of the anatomic variations of the celiac axis and the hepatic arteries is invaluable. A thorough preoperative analysis of the vascular system of the donor and recipient is a prerequisite for transplant surgery [4].
Materials and Methods
Two hundred patients who underwent abdominal CT angiography from July 2014 till July 2015 were retrospectively studied for hepatic arterial and celiac trunk anatomical variation. All the patients underwent multidetector abdominal CT angiography in a 64 slice Siemens Somatom machine. Institutional ethical committee approval was obtained. Being a retrospective study, informed consent was not obtained. Definitions of CHA, ambiguous celiac axis, course and division patterns of CHA, replaced hepatic artery, accessory hepatic artery were used as proposed by Song et al., [3]. The pattern of the aortic origin of branches of celiac axis, common hepatic artery and its branches was analyzed.
CT examination protocol
MDCT abdominal angiography was done using Siemens 64 slice CT machine by injecting intravenous non ionic iodinated contrast (with iodine concentration of 350mg/ mL) of 100 to 150ml at the rate of 3-5ml/kg with power injector (Imaxeon). Arterial phase images were obtained 11-17 seconds after the descending aorta enhancement to 100HU with use of a bolus tracking technique. Axial raw data base was acquired with 64 slice Siemens Somatom definition-AS with the Slice thickness of 1x1mm and recon interval 1x.8mm. The initial non enhanced images were obtained followed by the contrast study. The CT images were obtained in a cranio-caudal direction. Maximum intensity projection (MIP) and volume rendered reconstructions were done for relevant cases.
Image interpretation
The raw axial images obtained from MDCT were processed on the workstation to obtain 3D reconstruction with maximum intensity projection (MIP) and volume rendering.
Image interpretation was done by two experienced radiologists independently with 8 years of experience in interpreting the CT angiographic images.
Definitions of arteries
CHA: Defined as the arterial trunk containing atleast one branch of the hepatic artery and the gastroduodenal artery.
Ambiguous Celiac axis: Congenital absence of CHA or congenital presence of an anastomatic channel connecting SMA and celiac axis.
Normal celiac axis: Arterial trunk giving rise to CHA, left gastric and splenic arteries.
Replaced right hepatic artery: RHA originating from an ectopic location (SMA).
Replaced left hepatic artery: LHA originating from LGA.
Accessory hepatic artery: Arterial origin from typical as well as ectopic location.
CHA trifurcation: CHA trifurcating into RHA, LHA and GDA.
Double hepatic arteries: Early branching of RHA and LHA from the celiac axis or when one or both the hepatic arteries originate from the aorta.
The anatomic origins of the celiac axis, the CHA, the splenic artery, left gastric artery and the superior mesenteric arteries were assessed. The images were re-analysed in case of any discrepancy between the two radiologists. We followed the nomenclature system followed by Song et al., and Michel for the celiac axis anatomy and its downstream hepatic arterial variation respectively [3,5] [Table/Fig-1,2 and 3].
Abbreviations used in the study.
| CHA | Common hepatic artery |
|---|
| HAP | Hepatic artery proper |
| SMA | Superior mesenteric artery |
| LGA | Left gastric artery |
| RHA | Right hepatic artery |
| LHA | Left hepatic artery |
| MHA | Middle hepatic artery |
| GDA | Gastroduodenal artery |
| SpA | Splenic artery |
| HM trunk | Hepatomesenteric trunk |
| HSp trunk | Hepatosplenic trunk |
| GSp trunk | Gastrosplenic trunk |
| CM trunk | Celiacomesentric trunk |
| HSpM trunk | Hepatosplenomesentric trunk |
Celiac Axis Variations in 5002 Patients by Song et al., [3].
| Celiac Axis Anatomy Type | No. of Patients (n = 5002)(Percentage in parenthesis) |
|---|
| Normal anatomy * | 4457 (89.1) |
| Anatomic variation | 482 (9.6) |
| HSp trunk + LG + SM | 221 (4.42) |
| HM trunk + GSp trunk | 132 (2.64) |
| CM trunk | 53 (1.06) |
| HSpM trunk + LG | 34 (0.68) |
| HM trunk + LG + Sp | 12 (0.24) |
| CH + GSp trunk + SM | 11(0.22) |
| HG trunk + SpM trunk | 8 (0.16) |
| CH + LG + Sp + SM | 5 (0.10) |
| CH + GSpM trunk | 3 (0.06) |
| CH + LG + SpM trunk | 1 (0.02) |
| HG trunk + Sp + SM | 1 (0.02) |
| HSp trunk + GM trunk | 1 (0.02) |
| HGM trunk + Sp | 0 |
| CH + GM trunk + Sp | 0 |
| Ambiguous anatomy | 63 (1.26) |
Michel’s hepatic artery variants classification [5].
| Type | Percentage |
|---|
| I Standard Anatomy | 60(55-61) |
| II Replaced LHA | 7.5(3-10) |
| III Replaced RHA | 10(8-11) |
| IV Replaced RHA and LHA | 1 |
| V Accesory LHA from LGA | 10(8-11) |
| VI Accesory RHA from SMA | 5(1.5-7) |
| VII Accessory RHA and LHA | 1 |
| VIII Accessory RHA and LHA and replaced LHA or RHA | 2.5 |
| IX CHA replaced to SMA | 3(2-4.5) |
| X CHA replaced to LGA | 0.5 |
| Unclassified | |
| o CHA separate origin from aorta | 2 |
| o Double hepatic artery | 4 |
| o PHA replaced to SMA and GDA origin from aorta | <0.5 |
Results
The celiac axis (CA) and the hepatic artery (HA) variations were analysed as per criteria laid by Song et al., and Michel [3,5]. A normal CA was seen in 179(89.5%) patients [Table/Fig-4]. Out of 15 possible CA variations, 5 types of celiac artery variations were seen in 14 patients. In the remaining 7 patients, the CA anatomy was classified as ambiguous since there was separate origin of the right and left hepatic arteries from the CA with absent common hepatic artery (CHA). The CHA originated normally from the celiac axis in 94% of the cases. Variation of CHA origin was seen in 5 patients. Normal HA anatomy was seen in 114 (57%) patients. Variation in HA anatomy was seen in 86 (43%) patients [Table/Fig-5]. Origin of the right hepatic artery (RHA) from the hepatic artery proper was seen in 182 (91%) patients and replaced origin of RHA from the superior mesenteric artery (SMA) was seen in 18 (9%) of the cases. Accessory RHA was seen in 7 (3.5%) patients. The left hepatic artery (LHA) originated from the hepatic artery proper in 186 (93%) patients and replaced origin of LHA from the left gastric artery (LGA) was found in 14 (7%) patients. Accessory left hepatic artery was found in 22 (11%) cases. Double hepatic artery seen in 7 (3.5%) patients. CHA replaced to LGA was seen in 1 patient (0.5%). CHA trifurcation was seen in 11 (5.5%) patients. CHA was replaced to SMA in 4 (2%) cases.
Results in our study (Using the classification of Song et al.)
| Celiac Axis Anatomy Type | No. of Patients (n = 200) |
|---|
| Normal anatomy | 179(89.5) |
| Anatomic variation | 21 (10.5%) |
| HSp trunk + LG + SM | 7 (3.5) |
| HM trunk + GSp trunk | 3 (1.5) |
| CM trunk | 0 |
| HSpM trunk + LG | 2 (1) |
| HM trunk + LG + Sp | 1(0.5) |
| CH + GSp trunk + SM | 0 |
| HG trunk + SpM trunk | 0 |
| CH + LG + Sp + SM | 0 |
| CH + GSpM trunk | 0 |
| CH + LG + SpM trunk | 0 |
| HG trunk + Sp + SM | 0 |
| HSp trunk + GM trunk | 1 (0.5) |
| HGM trunk + Sp | 0 |
| CH + GM trunk + Sp | 0 |
| Ambiguous anatomy | 7 (3.5) |
| Hepatosplenic trunk replaced to left gastric artery | 1(0.5) |
Results in our study (Using the Michel’s hepatic artery classification):
| Type | No: of cases | Percentage |
|---|
| I Standard Anatomy | 114 | 57 |
| II Replaced LHA | 12 | 6 |
| III Replaced RHA | 16 | 8 |
| IV Replaced RHA and LHA | 2 | 1 |
| V Accesory LHA from LGA | 17 | 8.5 |
| VI Accesory RHA from SMA | 2 | 1 |
| VII Accessory RHA and LHA | 2 | 1 |
| VIII Accessory RHA and LHA and replaced LHA or RHA | 3 | 1.5 |
| IX CHA replaced to SMA | 3 | 1.5 |
| X CHA replaced to LGA | 1 | 0.5 |
| Unclassified | | |
| o CHA separate origin from aorta | 0 | 0 |
| o Double hepatic artery | 7 | 3.5 |
| o PHA replaced to SMA and GDA origin from aorta | 0 | 0 |
Discussion
Anatomic variations of the celiac axis and superior mesenteric artery occur due to anomalous embryogenesis of primitive ventral blood vessels originating from the abdominal aorta [6].
Rapid volumetric acquisition of thin-slice high resolution images of the abdominal arteries during the phase of maximal contrast enhancement with the help of MDCT allows 3D reconstructions to be created, providing the radiologist and the surgeon with a 3D model of the patient’s arterial anatomy. MDCT angiography has a reported accuracy of 97–98% compared with conventional angiography for detecting arterial variants [7].
Song et al., studied common hepatic artery (CHA) and celiac axis (CA) variations in 5002 patients and found out 13 types of CA variation out of 15 possible types. They noted normal CA in 89.1% and 13 types of CA variation identified in 9.4% of the people. In the remaining 1.26%, the CA anatomy was classified as ambiguous because the CHA was absent owing to separate origin of the hepatic arteries [3].
Song et al., found that the hepatosplenic trunk with separate origin of the left gastric artery from the aorta (4.42%) is the commonest CA variation followed by the gastrosplenic trunk (2.86%).
Sureka et al., in their study in 600 patients, found out six types of celiac axis anatomic variations and 91% of the patients had normal celiac axis anatomy [8]. They also identified that the most common celiac axis variation in their study were the hepatosplenic trunk (2.83%) and the gastrosplenic trunk (~1.4%). Ugurel et al., in their study found out that the gastrosplenic trunk was the most prevalent variation (4%), followed by the hepatosplenic trunk (3%) [9].
In our study, there were 5 types of celiac axis variation identified in 14 patients, with normal celiac axis anatomy [Table/Fig-6] in 179 (89.5%) patients. The hepatosplenic trunk (4%) [Table/Fig-7] was the most common celiac artery variation with separate origin of left gastric artery (LGA) and superior mesenteric artery (SMA) followed by gastrosplenic trunk and a common hepatomesenteric trunk (1.5%) [Table/Fig-8].
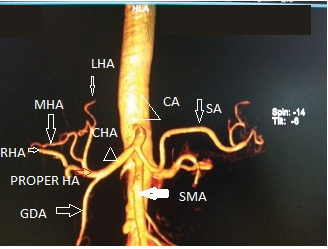
CT angio VRT image showing normal anatomy of celiac axis and CHA.
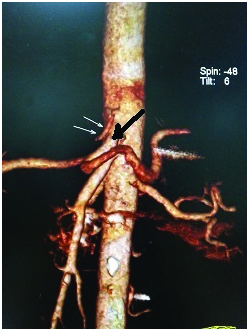
CT angio VRT image showing the separate origin of LGA (double arrow) from aorta and the hepatosplenic trunk (black arrow).
Gastrosplenic trunk with separate origin of SMA and CHA from aorta was not found in our study which was found in 0 .22% and 0.83% in the studies of Song et al and Sureka et al., respectively.
Hepatomesenteric trunk with gastrosplenic trunk was seen in 3 (1.5%) [Table/Fig-8] patients and hepatomesenteric trunk with separate origin of splenic and left gastric artery was seen in 1 patient (0.5%) [Table/Fig-9]. This variation in our study is reasonably comparable to results in Song et al., where common hepatomesenteric trunk with gastrosplenic trunk was seen in 2.64% and common hepatomesenteric trunk with separate origin of left gastric and splenic artery was found in 0.24%.
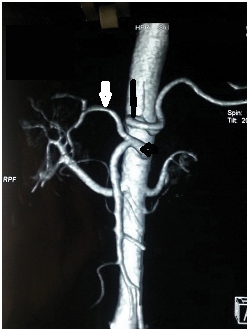
CT angio volume rendered image showing hepatomesenteric trunk /common hepatic artery arising from the superior mesenteric artery (horizontal black arrow) and gastrosplenic trunk (vertical black arrow).
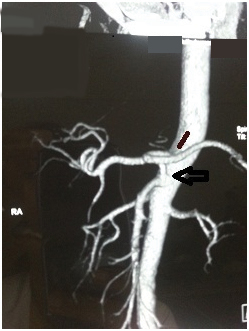
CT angio volume rendered reconstructed image showing the hepatomesenteric trunk/common origin of common hepatic artery and superior mesenteric artery (large black arrow) with separate origin of splenic artery and left gastric artery(small black arrow).
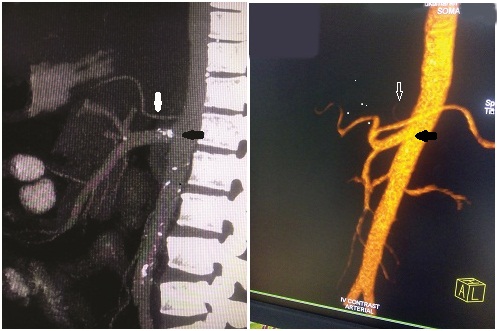
We also reported a rare variant hepatosplenomesenteric trunk with separate origin of left gastric artery [Table/Fig-10a,b] from the aorta in two cases which were comparable to the results of Song et al., [3].
(a) CT angio reconstructed sagiital maximum intensity projection showing a rare celiac axis variant hepatosplenomesenteric trunk(black arrow) with separate origin of left gastric artery(white arrow). (b) Reconstructed volume rendered saggital section showing the same-hepatosplenomesentric trunk (black arrow) with separate origin of left gastric artery (white arrow).
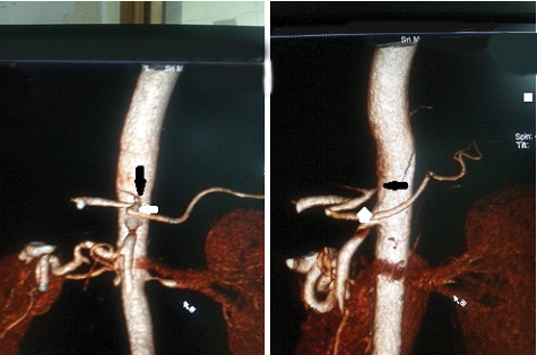
We reported another rare variant where the hepatosplenic trunk was replaced to left gastric artery which was directly arising from the aorta [Table/Fig-11a,b].
CT angio VRT image showing a rare variant where left gastric artery is arising (black arrow) from aorta and giving rise to hepatosplenic trunk (white arrow).
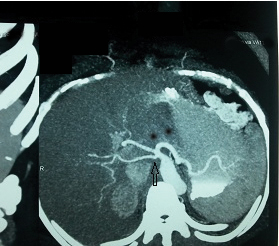
Ambiguous celiac axis anatomy [Table/Fig-12] due to separate early origin of right and left hepatic anatomy from the celiac axis was found more frequently (3.5%) in our study whereas it was seen in 1.26% and 2.1% in the studies of Song et al and Sureka et al., respectively. Ambiguous anatomy due to persistent anastomotic channel between celiac artery and superior mesenteric artery was not found in our study.
CT angio maximum intensity projection axial image showing celiac artery trifurcation with direct early origin of right and left hepatic artery (vertical black arrow) from the celiac axis.
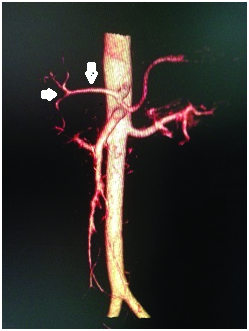
Sureka et al., in their study in 600 patients found the CHA arising normally from celiac axis in 95.83% of the patients. Variations in anatomic origin of CHA were seen in 8 patients.
We found that the CHA originated normally from the celiac axis in 94% of the cases. Variation of CHA origin was seen in 5 patients. Most commonly, it originated from the SMA in 3 patients (1.5%) [Table/Fig-8] which is comparable with the results obtained by Sureka et al., Separate origin of CHA from aorta which was seen in 0.33% by Sureka et al., was not found in our study, whereas we reported a rare variant where CHA was replaced to LGA originating from aorta in 1 patient(0.5%) [Table/Fig-11a,b]. Ambiguous anatomy due to hepatomesenteric trunk [Table/Fig-9] where there is a common origin of common hepatic artery and the superior mesenteric artery from the aorta was found in 1 patient. Ambiguous anatomy due to the absent CHA with celiac axis trifurcation giving rise to double hepatic artery was seen in 3.5% of the patients [Table/Fig-12]. CHA trifurcation [Table/Fig-13] into right, left hepatic arteries and gastroduodenal artery was seen 11(5.5%) of the patients. These results in our study are comparable with the results (2.1%) obtained by Sureka et al., [8].
CT angio volume rendered reconstructed image showing the common hepatic artery trifurcating into gastroduodenal, right and left hepatic arteries.
Michel described 10 variant subtypes while classifying the hepatic artery anatomy [5]. His work also described the accessory and the replaced hepatic arterial systems.
Chen et al., and Kamel et al., reported a normal hepatic artery anatomy in 68% and 70% of patients respectively using digital subtraction angiography [10,11]. In our study, normal hepatic arterial anatomy (Michel’s type I, [Table/Fig-6]) was present in 57% (114) cases which correlate well with the results of Michel.
The most commonly reported anatomic variation is Type III (replaced right hepatic artery), present between 6% and 15.5% [12,13] followed by, Type II (replaced left hepatic artery) in 2.5–10% [13,14]. In our study, the most common type is Type V (accessory left hepatic artery [Table/Fig-14] from left gastric artery which is present in 8.5% of the cases. This finding was similar to that of Erbay et al., [15]. This was followed by Type III [Table/Fig-14] and Type II [Table/Fig-15,16] which are present in 8% and 6% of the cases respectively which correlated with the prevalence range in Michel’s classification.
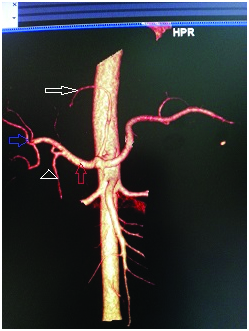
CT angio volume rendered reconstructed image showing accessory left hepatic artery(white arrow) arising from the left gastric artery and the common hepatic artery (red arrow) dividing into right, middle and left hepatic arteries(blue arrow) after giving rise to gastroduodenal artery(arrow head).
CT angio volume rendered image showing replaced right hepatic artery (pink arrow) from the superior mesenteric artery (black arrow).
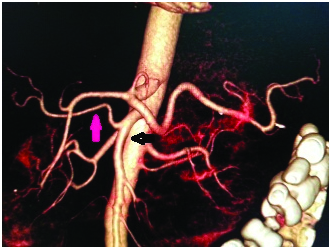
CT angio volume rendered reconstructed image showing replaced left hepatic artery (red arrow) arising from the left gastric artery and the common hepatic artery (white arrow) bifurcating into right hepatic artery (arrow head) and gastroduodenal artery (blue arrow).
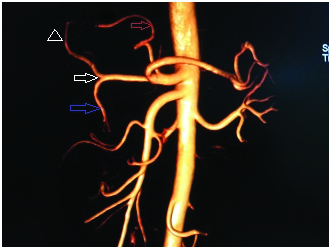
Types VI, VII, VIII and IX and X are very rare anatomic variants. In our study, type IV, VI, VII are reported in 1% of the cases and Types VIII and IX reported in 1.5% and Type X reported in 0.5% of the cases.
Conclusion
Celiac axis and hepatic arterial anatomy is subject to numerous anatomic variations. Our study identified the variations in celiac axis and hepatic arterial anatomy in a sample of South Indian population using established classifications. Our results correlated well with studies in other populations. Adequate knowledge of these variations would be of great help to the interventional radiologist and hepatobiliary surgeon.