Oral Lichen Planus (OLP) is a common chronic immunological inflammatory mucocutaneous disorder affecting 0.5 – 4% of the adult population with a higher prevalence in middle-aged females [1]. Pain and soreness are common symptoms which hinders oral function. A hypothesis has been proposed in relation to pathogenesis of OLP, involving both antigen-specific and non-specific mechanisms. Antigen presentation by basal keratinocytes and antigen-specific keratinocyte killing by CD8 cytotoxic cells are taken into consideration in antigen-specific mechanisms while in non-specific mechanisms mast cells degranulation and matrix metalloproteinase activation is involved [2]. These mechanisms might cause T cell accumulation in the superficial lamina propria causing basement membrane disruption and intraepithelial T cell migration and finally keratinocyte apoptosis [3]. There is also evidence that in certain populations, viruses (hepatitis C) may be important in the immunopathogenesis of OLP, but the most accepted one is the immunopathogenesis of OLP, specifically involving the cellular arm of the immune system [4]. OLP can manifest itself in different clinical forms both asymptomatic and symptomatic. The keratotic reticular, papular, plaque- like white patches is often present without any signs or complaints-painless. Erosive atrophic and ulcerative lesions, which are surrounded by keratotic forms manifest damage epithelium. They are painful with or without burning sensation, thus interfering with eating, speaking, swallowing [5]. Cutaneous lesions typically present as small pruritic, white to violaceous flat-topped papules. It is more common in women with ratio of F:M – 3:2, with a peak incidence at the age of 30 to 60 years [6]. OLP may be present anywhere in the oral cavity and usually occurs bilaterally/symmetrically on the buccal mucosa, more rarely on lateral borders of the tongue and the gingiva. Desquamative gingivitis may be diagnosed when it presents itself on gingiva as atrophic/erosive forms [7]. The most characteristic feature is the presence of white striations known as Wickham striae which form a lacy network and are more commonly seen bilaterally on the buccal mucosa. The symptoms arising from OLP vary. The ulcerative form more commonly gives rise to pain and soreness and interferes with oral function too [8].
OLP can be associated with considerable morbidity and altered quality of life, especially when patients suffer from ulcerative lesions [9].
The management of OLP is a challenge for clinicians. Therapeutic management of extensive disseminated and especially erosive OLP can be challenging for both the patient and the oral physician.
The mainstream of treatment modalities consist of topical and systemic corticosteroids, retinoids, topical Interferon-α cream, circuminoids, photochemotherapy, antimalarials, dapsone, immunosuppressive agents like cyclosporine, cyclophosphamide, and azathioprine. OLP is a chronic disease and requires long-term corticosteroids use which has adverse effects. Hence, a safer and more effective therapy for symptomatic OLP is necessary [10].
A promising new treatment for OLP is the topical form application of 0.2% hyaluronic acid. Hyaluronic acid (HA) was discovered in bovine vitreous humor by Meyer and Palmer in 1934 [11]. It is most frequently referred to as HA due to the fact that it exist invivo as a polyanion and not in the protonated acid form. HA is universally present and distributed widely in vertebrates and is a component of the cell coat of numerous strains of bacteria [12].
Literature review suggests that there are a very few studies available with 0.2% hyaluronic acid in the treatment of OLP, hence the present study is an attempt to evaluate the effectiveness of hyaluronic acid in the management of OLP.
Materials and Methods
This study was conducted in the Oral Medicine Department, S.D.M. college of Dental Sciences, Dharwad.
A total of 50 patients with symptomatic lesions and histologically proven cases of Oral lichen planus were selected at random from among the patients attending the outpatient section of Department of Oral Medicine. A detailed case history of the patient and a thorough clinical examination was recorded on a standard proforma.
Inclusion criteria: Patients above 18 years of age suffering from symptoms of histopathologically proven oral lichen planus and who were willing to participate in the study.
Exclusion criteria: Patients with known history of hypersensitivity to hyaluronic acid. Subjects with diabetes mellitus, hypertension, or on systemic steroid therapy, immunosuppression either due to disease or immunosuppressant drugs, regularly on anti-oxidants, immunomodulators or anti-rheumatic drugs for the past one month.
Drug preparation: HA was procured in pure powder form manufactured by Cadila Pharmaceuticals Pvt Ltd.
0.2% w/w - 0.2 gm of drug in 100 gm w/w of vehicle.
Two grams of drug was weighed by an electronic weighing machine and was mixed with 1000 gms of vehicle to make 1kg of 0.2% hyaluronic acid orabase which was then dispensed in individual 5 gm small plastic containers.
The Placebo was prepared by a pharmacist at the pharmacology section. It contained carboxymethyl cellulose and 0.1% sodium benzoate.
The patients were advised to apply the drug regularly three times daily after food. They were advised to apply drug over the affected site with their index finger in the mouth and allow it to remain for 30 minutes. They were also instructed not to eat, drink or smoke for a minimum of 30 minutes after the application of the drug. The patients were instructed to make a note in a diary if they skipped the dose on any particular day and the reason for it.
The intra-oral site was recorded and the clinical appearance of the lesion- whether it was the reticular, erosive, ulcerative/bullous, plaque or papular form, was noted. The severity of pain was assessed using the Visual Analogue Scale (VAS) and patients were made to rate their pain on a scale of 0 to 10, with 10 being the most severe and the intensity score for erythema was measured by using Modified oral mucositis index (0-3). The degree of erythema was graded as mild, moderate and severe [13]. Finally the size of the lesions was recorded in length into breadth in cm2 by measuring from the maximum points on the spread of the lesions [Table/Fig-1].
Flexible, transparent sheet (intraoral grid) divided into calibrations of one mm square used for measurement of the lesions.

The selected patients were divided randomly into two groups. Group-I comprising of 25 patients received topical 0.2% hyaluronic acid for 14 days. Group-II comprising of 25 patients received topical placebo for 14 days. After Day 14 no treatment was given to patients in both the groups and was followed up for the subsequent two weeks. Patient evaluation was done at the end of each week of therapy, first and second week of follow-up.
Follow-up Evaluations: Patients were then recalled to the department for a clinical examination on Day 7, Day 14, Day 21 and Day 28 following the onset of treatment. On every follow-up evaluation, the clinical status was recorded in their proforma. During these visits, any changes in the VAS values, and site of lesion, colour, size, or appearance of the lesions were duly noted on the proforma. On every visit the size and appearance of the lesions were evaluated by intraoral measurements with the help of flexible, transparent sheet (intraoral grid) divided into calibrations of one mm square. The entire data was then entered into the proforma [Table/Fig-2]. The procedures followed were in accordance with the ethical standards committee on human experimentation and with the Helsinki Declaration of 1975 that was revised in 2000.
Patient Proforma used for measurement and assessment of the lesions.
| Pre op | Day 7 | Day 14 | Day 21 | Day28 |
|---|
| VAS (1-10) | | | | | |
| CLINICAL ASSESSMENT |
| Site | | | | | |
| Appearance | | | | | |
| Degree of Erythema | | | | | |
| Size of the lesion-Length | | | | | |
| Size of the lesion-Breadth | | | | | |
| Total area | | | | | |
Statistical Analysis
Comparison of test/study group and control group with VAS and degree of erythema was done by Mann-Whitney U test. Comparison of test/study and control with area scores was done by t-test. Comparison of time periods with VAS scores in test group and control group and with Degree of erythema scores in test group and control group was done by Wilcoxon matched pairs test by ranks. Comparison of time periods with area scores in test group and control group was done by paired t-test.
Results
The details of the participants in the study and the mean baseline scores are presented in [Table/Fig-3]. All the patients did comply to the follow up and the results were evaluable. There was no significant difference noted between the two groups regarding age, sex distribution and types.
Age and Sex distribution in study and control groups.
| HA (test group/group-1) | Placebo (control group/group-2) |
|---|
| Total no. of patients | 25 | 25 |
| Males | 13 | 11 |
| Female | 12 | 14 |
| Age range (years) | 19-75 | 26-70 |
| Mean baseline scores of VAS | 7.08 | 7.96 |
| Degree of Erythema baseline mean scores | 1.96 | 1.64 |
| Size of the ulcerated or erosive area (± SD) baseline mean scores | 8.28 | 8.67 |
| Type of OLP |
| Reticular | 19 | 20 |
| Erosive | 2 | 4 |
| Combined | 0 | 0 |
| Desquamative gingivitis | 1 | 0 |
| Plaque | 0 | 0 |
| Pigmented | 1 | 0 |
| Atrophic | 2 | 1 |
Symptomatic effect of soreness evaluation- there was a significant reduction in VAS scores in the test group as compared to placebo- [Table/Fig-4].
Comparison of study and control group with respect to VAS scores on Preoperative day, DAY 7, DAY 14, DAY 21 and DAY 28
| Time | Group | N | Mean | SD | SE | Sum of Ranks | U-value | Z-value | p-value | Significance |
|---|
| PREOP | Test | 25 | 7.08 | 1.53 | 0.31 | 521 | 196.000 | -2.3131 | 0.0207 | * |
| Control | 25 | 7.96 | 1.14 | 0.23 | 754 | | | | |
| DAY 7 | Test | 25 | 4.48 | 2.42 | 0.48 | 408.5 | 83.5000 | -4.4868 | <0.001 | ** |
| Control | 25 | 7.72 | 1.40 | 0.28 | 866.5 | | | | |
| DAY 14 | Test | 25 | 2.88 | 1.88 | 0.38 | 333 | 8.0000 | -5.9483 | <0.001 | ** |
| Control | 25 | 7.68 | 1.38 | 0.28 | 942 | | | | |
| DAY 21 | Test | 25 | 1.96 | 1.43 | 0.29 | 325.5 | 0.5000 | -6.1004 | <0.001 | ** |
| Control | 25 | 7.76 | 1.39 | 0.28 | 949.5 | | | | |
| DAY 28 | Test | 25 | 1.48 | 1.42 | 0.28 | 325 | 0.0000 | -6.1137 | <0.001 | ** |
| Control | 25 | 7.76 | 1.39 | 0.28 | 950 | | | | |
p<0.05- Significant- *, p<0.01-Highly significant-**
p>0.05- Not Significant. (NS)
Similarly, there was also significant reduction in the degree of erythema, change in the size of the lesion and area of the lesion in the test group [Table/Fig-5,6].
Comparison of study and control with respect to Degree of Erythema on Preoperative day, DAY 7, DAY 14, DAY 21 and DAY 28. (Mann-Whitney U Test)
| Time | Group | N | Mean | SD | SE | Sum of Ranks | U-value | Z-value | p-value | Significance |
|---|
| PREOP | Test | 25 | 1.96 | 0.35 | 0.07 | 722.5 | 227.5000 | -2.1527 | 0.0313 | * |
| Control | 25 | 1.64 | 0.64 | 0.13 | 552.5 | | | | |
| DAY 7 | Test | 25 | 1.04 | 0.61 | 0.12 | 552 | 227.0000 | -1.8594 | 0.0630 | NS |
| Control | 25 | 1.40 | 0.71 | 0.14 | 723 | | | | |
| DAY 14 | Test | 25 | 0.64 | 0.64 | 0.13 | 478 | 153.0000 | -3.3688 | 0.0008 | ** |
| Control | 25 | 1.32 | 0.63 | 0.13 | 797 | | | | |
| DAY 21 | Test | 25 | 0.52 | 0.59 | 0.12 | 451 | 126.0000 | -3.9141 | 0.0001 | ** |
| Control | 25 | 1.36 | 0.70 | 0.14 | 824 | | | | |
| DAY 28 | Test | 25 | 0.48 | 0.59 | 0.12 | 443.5 | 118.5000 | -4.0537 | 0.0001 | ** |
| Control | 25 | 1.36 | 0.70 | 0.14 | 831.5 | | | | |
p<0.05- Significant- *, p<0.01-Highly significant-**
p>0.05- Not Significant. (NS)
Comparison of study and control with respect to Area scores on Preop DAY, DAY 7, DAY 14, DAY 21 and DAY 28 by t-test.
| Time | Group | N | Mean | SD | SE | t-value | p-value | Significance |
|---|
| PREOP | Test | 25 | 8.28 | 6.76 | 1.35 | -0.2310 | 0.8190 | NS |
| Control | 25 | 8.67 | 5.32 | 1.06 | | | |
| DAY 7 | Test | 25 | 5.40 | 4.20 | 0.84 | -2.4530 | 0.0180 | * |
| Control | 25 | 8.71 | 5.28 | 1.06 | | | |
| DAY 14 | Test | 25 | 4.15 | 3.50 | 0.70 | -3.5790 | 0.0010 | ** |
| Control | 25 | 8.69 | 5.30 | 1.06 | | | |
| DAY 21 | Test | 25 | 3.10 | 2.27 | 0.45 | -4.9330 | <0.001 | ** |
| Control | 25 | 8.75 | 5.26 | 1.05 | | | |
| DAY 28 | Test | 25 | 2.59 | 1.76 | 0.35 | -6.0140 | <0.001 | ** |
| Control | 25 | 9.05 | 5.08 | 1.02 | | | |
p<0.05- Significant- *, p<0.01-Highly significant-**
p>0.05- Not Significant. (NS)
A highly statistically significant difference was seen between study and control group when VAS was compared at the end of Day 7, Day 14, Day 21 and Day 28.
Statistically no significant difference was seen between study and control group when degree of erythema was compared at the end of Day 7.
A highly statistically significant difference was seen between study and control group when degree of erythema was compared at the end of Day 14, Day 21 and Day 28.
Statistically no significant difference was seen between study and control group when the mean area of the lesion was compared preoperatively.
Statistically significant difference was seen between study and control group when the mean area of the lesion was compared at the end of Day 7.
A highly statistically significant difference was seen between study and control group when the mean area of the lesion was compared at the end of Day 14, Day 21 and Day 28.
In our study in the study/drug group, in 8 patients the VAS score reduced to 0. This indicated that the pain or the burning sensation to hot and spicy foods disappeared completely. In 2 patients, the pain or the burning sensation to hot and spicy foods disappeared on Day 7 itself. These 2 patients were just one week into the treatment. In 2 patients the pain or the burning sensation to hot and spicy foods disappeared on Day 14 whereas, in another patient the pain or burning sensation disappeared on Day 21. In 5 patients, the pain or the burning sensation to hot and spicy foods reduced substantially. These patients had severe burning sensation to hot and spicy foods before the start of treatment. The VAS scores reduced considerably in these patients. This showed that all patients had substantial decrease in their pain and burning sensation or VAS scores. All the patients showed a constant decrease in their VAS scores on every follow up appointments.
In the placebo group/control group, in 6 patients the burning sensation to hot and spicy foods or the VAS score reduced very minimally. In 3 patients, there was actual mild increase in the burning sensation to hot and spicy foods. The above results of VAS scores showed that in the control group of patients/placebo group, most of the patients did not show any significant reduction in the pain and burning sensation to hot and spicy foods.
Signifying the healing effects of HA there was a remarkable reduction in the degree of erythema in the study group. The duration of reduction did vary among patients, and in four subjects the changes were evident in just seven days of onset of therapy. The results clearly showed that in all the patients either the degree of erythema reduced to mild form or changed to normal mucosa [Table/Fig-7,8], except in 1 patient.
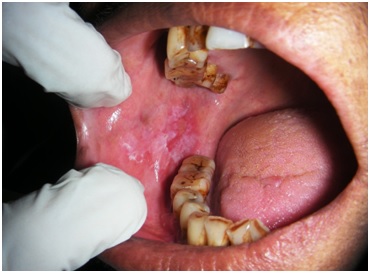
Oral Lichen Planus affecting right buccal mucosa- Before treatment.
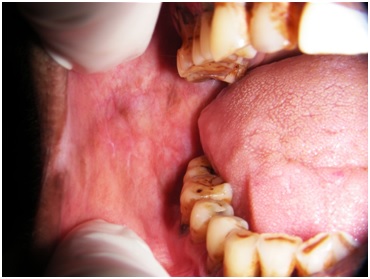
Photograph of the same patient after 4 weeks treatment with topical 0.2% hyaluronic acid.
In the placebo group there was no significant reduction in the degree of erythema and it remained unchanged in most of the patients.
Similarly, there was considerable reduction in mean area of the lesions in the HA group. It was also noted that there was no recurrence of lesions even after the cessation of therapy.
Discussion
The management of OLP is a challenge for clinicians. Patients must be advised that lichen planus is characterized by unpredictable exacerbations because of stress [14] that may, in some cases require treatment for many years.
The medical line of therapy is aimed at eliminating atrophic and ulcerative lesions, alleviating symptoms, and potentially decreasing the risk of malignant transformation [15].
Inspite of being the first-line therapy, new therapeutic modalities are needed due to the adverse effects of topical corticosteroids. OLP is a chronic disease and requires long-term corticosteroids use. Hence, there is a need for more effective therapy for symptomatic OLP.
A promising new treatment for OLP is the topical form application of 0.2% hyaluronic acid [1]. Hyaluronic acid (HA) was discovered in bovine vitreous humor by Meyer and Palmer in 1934 [11]. The precise chemical structure of HA, which contains repeating units of d-glucoronic acid and N–acetyl d-glucosamine, was first determined by Weissman and Meyer in 1954. It is most frequently referred to as HA due to the fact that it exists in vivo as a polyanion and not in the protonated acid form.
The synovial fluid, umbilical cord, skin and rooster comb, or from bacteria through a process of fermentation or direct isolation are the sources of commercially produced HA. Studies on the properties of HA like chemical and physicochemical and on its physiological role in humans have been done extensively [16]. HA is an ideal biomaterial for cosmetic, medical and pharmaceutical applications due to its versatile properties such as its biocompatibility, non-immunogenicity, biodegradability and viscoelasticity. A variety of mechanisms of the tissue healing properties of HA have been identified [17–19]. The healing potential of topical HA has been confirmed by clinical studies.
Topical HA has been tried in the management of recurrent aphthous stomatitis (RAU). Nolan et al., in their study did evaluate the efficacy in patients with RAU [20]. They inferred that there was a significant reduction in ulcer soreness with immediate reduction of symptoms.
HA has also been used to treat actinic keratoses [21]. It was also evident that topical form did improve wound healing in acute radio epithelitis [22], and venous leg ulcers [23] did positively respond to treatment.
There are a very few studies available with 0.2% hyaluronic acid in the treatment of OLP, hence an attempt has been made to study the effect of hyaluronic acid on OLP.
A study evaluating the efficacy of a topical hyaluronic acid (HA) gel preparation (0.2%) in the management of oral lichen planus was done by Nolan A et al. A significant reduction in soreness (upto 4 hours post application), and size of lesions were noted by them [1]. In this study, there was a significant reduction in the VAS scores, degree of erythema and size of the lesions in the HA group as compared to that of placebo. No significant changes in any of the parameters in the placebo group were found.
The results from the present study showed that the drug (0.2% hyaluronic acid) was effective in the management of lichen planus and the patients showed no exacerbation or recurrence of lesions even after cessation of treatment. The placebo although did have an effect on the degree of erythema but the extent of reduction was not in comparison with that of the drug.
Conclusion
In the present randomized controlled study topical hyaluronic acid 0.2% reduced the subjective symptoms of burning sensation to hot and spicy foods by showing statistically significant reduction in the VAS. Statistically significant improvements were observed in the objective criteria which involved the degree of erythema and the mean area of the lesions with 0.2% hyaluronic acid application than compared to the control group on placebo.
Patients did not report any adverse effects like local irritation or any discomfort during the two weeks of application of 0.2% hyaluronic acid and during the subsequent two weeks of follow up.
Steroids are considered as a gold standard for treatment of OLP but their long-term use has known to cause side effects. Hence, topical hyaluronic acid, which is relatively safer, can be considered as a treatment option for use on a long term basis.
p<0.05- Significant- *, p<0.01-Highly significant-**
p>0.05- Not Significant. (NS)
p<0.05- Significant- *, p<0.01-Highly significant-**
p>0.05- Not Significant. (NS)
p<0.05- Significant- *, p<0.01-Highly significant-**
p>0.05- Not Significant. (NS)