Chronic Obstructive Pulmonary Disease (COPD) is a chronic inflammatory disorder characterized by irreversible and progressive limitation of expiratory airflow. COPD is now considered to be a systemic disorder with multisystem involvement. The multisystem involvement along with the associated co-morbidities poses a challenge to the treating physicians [1]. Risk factors for development of COPD are a complex, involving both genetic and environmental factors and cigarette smoking is the most common aetiological agent.
Marked variation in prevalence of COPD may be attributed to differences in survey methods and diagnostic criteria’s used [2]. The estimated population prevalence of COPD (Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stage II and higher) is 5.3% - 14.9% and The Global Burden of Disease Study projected that COPD was ranked sixth as a cause of death in 1990, will become the third leading cause of death by 2020; a newer projection estimated COPD will be the fourth leading cause of death in 2030 [3]. In India, an estimate shows a prevalence of around 1.49 crore patients in the age group of 30 years and above. The prevalence rates in males ranges from 2.12% to 9.4% in studies conducted in north India to 1.4% to 4.08% in studies conducted in south India. This total number of cases is projected to be increased by 50% by the year 2016 [4].
COPD, usually associated with other co-morbidities which may have significant impact on the prognosis of the patient. Co-morbidities like Hypertension, osteoporosis, dyslipidaemia, diabetes, ischemic heart disease, chronic renal failure, heart failure, Anaemia, and polycythemia had been found to be associated with COPD [5,6]. Osteoporosis a major co-morbidity in COPD, is often under-diagnosed, and is associated with poor health status and prognosis. It is a well-known fact that vitamin D plays a major role in maintaining skeletal integrity. There has been increasing evidence in role of vitamin D in increasing risk of chronic diseases like cancer, autoimmune diseases, infectious diseases and cardiovascular diseases. Role of vitamin D in respiratory diseases is still need to be addressed. However literature shows lower levels of vitamin D was associated with reduction of lung function which was assessed by FEV1 and FVC in normal subjects. Vitamin D deficiency occurs frequently in COPD and correlates with severity of COPD.
But whether vitamin D deficiency has higher prevalence in COPD patients compared to general population is not well documented in India. In addition level of vitamin D in various combined COPD assessment stage has not been studied. The aim of the current study was to compare levels of vitamin D in COPD patients and healthy controls in south Indian population, and to further evaluate the associations between important COPD characteristics to levels of 25(OH) D, including the variables combined assessment stage, pack year and body mass index.
Materials and Methods
A case control study was conducted during the time period of November 2012 to July 2014 among 81 subjects with COPD. Sample size was derived after discussion with statistician with licensed software nMaster version 1.0. Patients who were suspected to have COPD on clinical history and met the criteria for COPD according to GOLD criteria [7] were included as cases in the study. Smoking was expressed as pack years. Age, gender and season matched controls without any conditions directly related to Vitamin D deficiency was taken as controls. Exclusion criteria include individuals with age > 65 years, BMI >30, Chronic kidney disease, History of small bowel resection, Cholestatic liver disease, Granulomatous disorder, individuals on treatment with Phenytoin, Phenobarbital, Carbamazepine, Isoniazid, Rifampin, Tenofovir, Efavirenz and individuals already on Vitamin D supplementation. All subjects underwent history taking, physical examination, questionnaire for Modified Medical Research Council Dyspnea scale [8], COPD Assessment Test, pulmonary function tests and blood test for Vitamin D levels. The study protocol was approved by the ethics committee of the Institution and informed consent was obtained prior to study entry.
Pulmonary Function Test
Pulmonary function testing was done in all patients. Forced vital capacity (FVC), forced expiratory volume (FEV1) and FEV1/FVC were measured using spirometry. Post bronchodilator reversibility testing using 400 μg of salbutamol was done to exclude patients with bronchial asthma.
Measurement of 25-hydroxyvitamin D
A 4 ml of serum samples in BD Vacutainer was collected and processed in biochemistry lab at kasturba hospital with chemiluminescence method by COBAS analyser. Patients were then classified based on their vitamin D levels into deficient, insufficient and normal when vitamin D levels are <20 ng/ml, 20-30 ng/ml, >30 ng/ml.
Statistical Analysis
Data analysis and interpretation was done with IBM SPSS Statistics v 20.0. Odds ratio was calculated with 95% confidence interval to compare the status of vitamin D with age and gender matched control. Categorical variables were expressed in number and percentage and were analysed using Fisher’s-exact test and Chi-square test. P<0.05 was taken as significant.
Results
A total of 162 patients took part in the study. Of these 81 individuals fulfilling the inclusion criteria were designated as cases and 81 Age, gender and season matched individuals without any conditions directly related to Vitamin D deficiency was taken as controls. Base line characteristics of both cases and controls are shown in [Table/Fig-1].
Base line characteristics.
| Cases | Controls |
|---|
| Numbers | 81 | 81 |
| Male | 75 | 75 |
| Female | 6 | 6 |
| Mean AGE | 56.93±8.6 y ears | |
| Smoker | 75 % | 49 % |
| BMI | | |
| < 18.5 | 20 % | 6% |
| 18.5-24.9 | 75% | 78% |
| ≥25 | 5% | 16% |
| Vitamin D | | |
| <20 | 47% | 33% |
| ≥20 | 53% | 67% |
| Mean value | 22.24 | 26.25 |
The maximum number of COPD patients (53/81) in this study was in the age group of 55-65 years with mean age 56.93(±8.6) years. In the present study majority of COPD patients 75 out of 81 (93%) were male.
COPD and Vitamin D
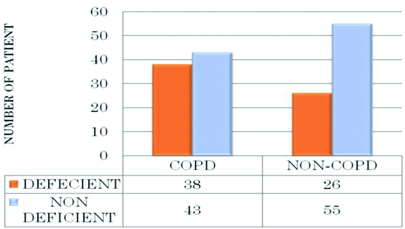
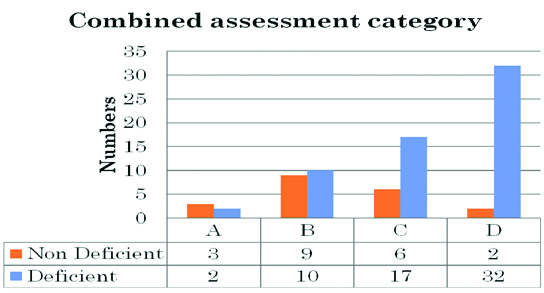
When correcting for season, age and gender the estimated risk for vitamin D deficiency was shown to be higher in COPD patients compared to controls [Table/Fig-2]. In COPD patients, there was a significant association between vitamin D levels and Combined COPD stage severity [Table/Fig-3].
Diagram shows vitamin D levels in COPD versus matched controls.
As per the secondary objective Combined COPD stage D was significantly associated with higher number of vitamin D deficient patients. Fisher’s-exact test 30.25 with degree of freedom 5 and p-value = 0.001.
Smoking and COPD
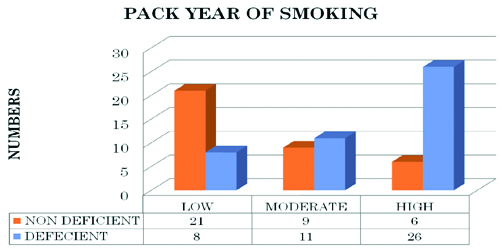
Smoking is a known risk factor for vitamin D deficiency, similar trend was observed in our study and the chance of having vitamin D deficiency is higher with increased pack year of smoking [Table/Fig-4].
As per the secondary objective Higher pack year was significantly associated with higher number of vitamin D deficient patients. Pearson chi-square with degree of freedom 18.28 and p-value = 0.001.
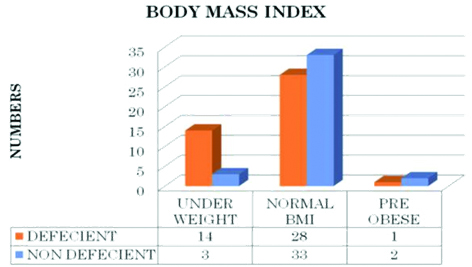
Low BMI was associated with high prevalence of Vitamin D deficiency in our study [Table/Fig-5].
As per the secondary objective lower BMI was significantly associated with higher number of vitamin D deficient patients. Fisher’s exact test 7.58 with degree of freedom 2 and p-value = 0.023.
Discussion
The maximum number of COPD patients (53/81) in this study was in the age group of 55-65 years with mean age 56.93 (±8.6) years, which is lesser to previous study. A similar study was carried out by Pearson et al., in Norway published in June 2012, wherein the mean age of presentation of patients with COPD was 63.5±9.8 years [9]. Patients between 55-65 years forms the maximum number of patients admitted, mainly because of the longer duration of smoking and repeated respiratory tract infections, which would have compromised their quality of life. As well patient age >65 years were excluded from the study as old age is a proven risk factor for Vitamin D deficiency. This explains the difference in mean age compared to study done by Pearson et al., where elderly patients were not excluded from the study.
In the present study majority of COPD patients 75 out of 81 (93%) were male. In a study by Pearson et al., 60% were male and 40% were female [9]. The BOLD data showed prevalence of COPD among men 11.8% and among women 8.5% [2].
When correcting for season, age and gender the estimated risk for vitamin D deficiency was shown to be higher in COPD patients compared to controls [Table/Fig-2]. In COPD patients, there was a significant association between vitamin D levels and Combined COPD stage severity [Table/Fig-3]. The above results are comparable with the study done by Pearson et al., [9]. In the NHANES III study, Black et al., found a dose- response relationship between 25(OH)D and both FEV1 and FVC, but no correlation with airway obstruction defined as a change in FEV1/FVC ratio [6]. However, in a recent study on COPD patients by Janssens et al., a strong relationship was found between GOLD stage and vitamin D deficiency suggesting a relationship with airway obstruction [10]. A similar association between 25(OH)D and FEV1 has been reported in adults with asthma [11]. These studies suggest towards a strong associations between COPD and vitamin D. However, causality could not be established.
Possible explanations for the increased prevalence of vitamin D deficiency in COPD include reduced capacity of aging skin to synthesize vitamin D, poor diet and low storage capacity of fat due to associated wasting [5]. Conversely, vitamin D deficiency also affects lung function by various mechanisms. Innate and adaptive immune systems are implicated in the pathogenesis of COPD [12]. Role of vitamin D in adaptive immunity is demonstrated by the fact that cells of adaptive immune system express receptor for vitamin D and require it for their optimal functioning [5]. Vitamin D has been shown to affect maturation of dendritic and TH1 cell [13]. The ability of 1,25(OH)2D to increase expression of antimicrobial peptides, and to decrease the expression of pro-inflammatory cytokines partly explains the association between vitamin D and increased susceptibility to respiratory infections [14]. All these leads to an increased rate of FEV1 decline [15]. In addition to these, vitamin D has been shown to be involved in tissue remodelling via fibroblast proliferation and collagen synthesis, and modulation of matrix metalloproteinase (MMP) levels [16]. Also, undiagnosed osteoporosis leads to vertebral compression, reduced rib cage mobility and hence a decline in pulmonary function [17].
Smoking is a known risk factor for vitamin D deficiency, similar trend was observed in our study and the chance of having vitamin D deficiency is higher with increased pack year of smoking [Table/Fig-4]. The results were comparable with the study by Cutillas-Marco E [18]. Cigarette smoke decreases the production of the active form of vitamin D (1,25-dihydroxy vitamin D) in lung epithelial cells [19]. Cigarette smoke may affect expression levels of the vitamin D receptor [20]. Vitamin D has immunomodulatory and anti-inflammatory effects. TNF-α, a key cytokine implicated in lung destruction of COPD, is down-regulated by vitamin D [21].
Low BMI was associated with high prevalence of Vitamin D deficiency in our study. Malnutrition and decreased skin thickness with decreased metabolism of Vitamin D explains the finding. In previous study obesity is also defined as a risk factor of Vitamin D deficiency but similar observation could not be made out due to limited sample size with high BMI [9].
Previous studies and our study showed that COPD patients had an increased risk for having vitamin D deficiency. Recently, a small RCT failed to demonstrate the role of vitamin D supplementation in reducing COPD exacerbation however RCT has been criticized for its smaller sample size [22].
Limitation
This was a cross sectional cross sectional study therefore inference of cause and effect is not possible. Although diet might be the minimal contributor to vitamin D status, lack of data on dietary intake is a limitation of study.
Conclusion
Vitamin D deficiency was observed in significant number of patients with COPD as compared to age, gender and season matched controls. Vitamin D deficiency was found in more than half of the study subjects, with increasing frequency across combined COPD class. Vitamin D deficiency was found more in subject with higher pack year of smoking and lower BMI.