Multiple Primary Dedifferentiated Liposarcoma of the Jejunal Mesentery: A Case Report and Review of Literature
Manu Vats1, Diwakar Pandey2, Himani Ahlawat3, Azaz Akhtar4, Nain Singh5
1 Post Graduate Student, Department of General Surgery, Lady Hardinge Medical College, New Delhi, India.
2 Post Graduate Student, Department of General Surgery, Lady Hardinge Medical College, New Delhi, India.
3 Post Graduate Student Department of Obstetrics and Gynaecology, Lady Hardinge Medical College, New Delhi, India.
4 Associate Professor, Department of General Surgery, Lady Hardinge Medical College, New Delhi, India.
5 Associate Professor, Department of General Surgery, Lady Hardinge Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Diwakar Pandey, C-702, Panchsheel Apartments, Plot No 24, Sector 4, Dwarka, New Delhi-110078, India.
E-mail: drdiwakarpandey86@gmail.com
Liposarcoma arising primarily from the intestinal mesentery is a rare malignancy. Malignancy is said to be synchronous when there is occurrence of two or more tumours that have not spread from a common site or recurred and show no evidence of metastasis. Multiple synchronous primary liposarcoma of the mesentery is a very unusual clinical finding. Here, we report a rare case of synchronous multiple primary dedifferentiated liposarcoma of jejunal mesentery in a 36-year-old female patient. Radiological investigations aided in making a provisional diagnosis of an ovarian malignancy. A staging laparotomy was performed and general surgeon’s help was sought due to the presence of three separate jejunal mesenteric masses of sizes 8x6 cms, 6x6 cms and 25x20 cms respectively. Complete excision of mesenteric masses with one feet of involved jejunum was done and a jejuno-jejunal anastomosis made. The histopathology report was indicative of multiple dedifferentiated liposarcoma of jejunal mesentery. Postoperatively patient received Doxorubicin, Dacarbazine and Ifosfamide based adjuvant chemotherapy in view of poorly differentiated tumour. Patient remains tumour free for the last 12 months of follow up.
Small bowel, Soft tissue sarcoma, Surgery, Synchronous
Case Report
A 36-year-old lady presented to the Department of Gynaecology with the complaint of dull aching pain and a lump in abdomen since the past 3 months. This was associated with constipation, loss of appetite and loss of weight. There was no history of any malignancy in the first degree relatives. General physical examination was essentially normal. On local examination, a lump involving the whole abdomen was palpable. It had an irregular surface, variable consistency and was dull on percussion. No free fluid was present clinically. Hepatosplenomegaly was absent. On per vaginum examination, the lower limit of the mass was felt through the anterior fornix. No mass was present in Pouch of Douglas. There was no history of any irregular menstrual cycles or bleeding per vaginum. The patient gives no history of any previous hospital admission or surgical procedure.
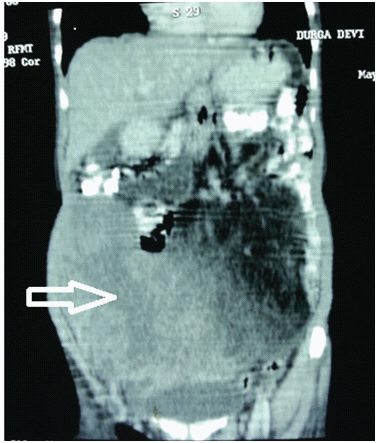
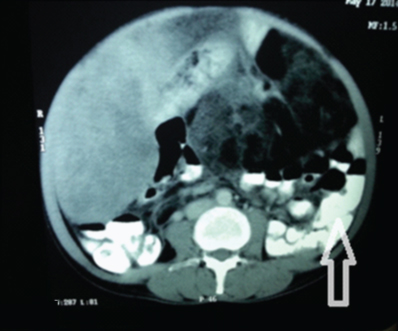
Biochemical investigations revealed CA-125 levels to be raised to 94.42 U/ml (normal <35U/ml). Serum Beta HCG and Alpha Foetoprotein were within normal limits. Chest radiograph was normal. PAP smear was negative for malignancy. Ultrasonography of abdomen and pelvis revealed a large, heterogeneous, hyper echoic abdomino- pelvic mass with hypo echoic areas within. Uterus was normal and both ovaries could not be visualized. A probable diagnosis of mucinous cystadenoma ovary was made. Contrast enhanced CT scan reported a large lobulated cystic cum solid mass seen extending from pelvis to epigastrium measuring 25cms x 20cms x 15cms [Table/Fig-1]. Heterogeneous enhancement of solid component and internal septation with fatty component on lateral side was seen. Bowel loops were displaced laterally and minimal ascites was present [Table/Fig-2]. CT scan failed to visualise bilateral ovaries separately, however the uterus was normal.
Coronal section CECT Abdomen. White arrow (red outline): Large lobulated cystic cum solid mass. Black arrow (green outline): Displaced bowel loops
Axial section. CECT Abdomen. Bowel loops were displaced laterally and minimal ascites was present
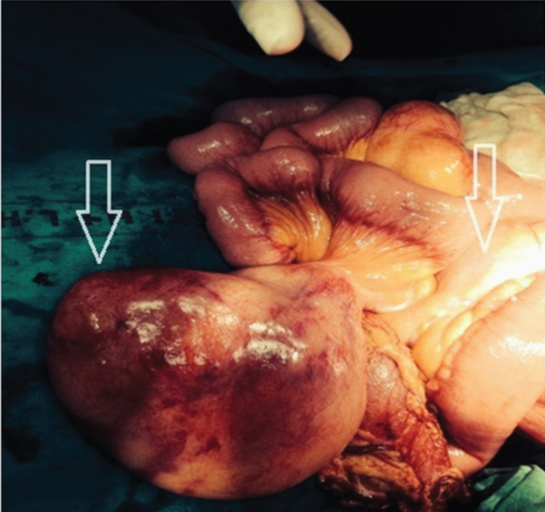
With a provisional diagnosis of an ovarian malignancy, keeping in mind the possibility of teratoma of ovary and mucinous cystadenoma, a staging laparotomy by lower midline vertical incision was carried out by the Gynaecologist. General Surgeon’s help was sought upon discovering the mesenteric masses. On exploration, three separate masses were identified. The first solid mesenteric mass measuring 8cms x 6cms, was 20cms distal to the Duodenojejunal junction (DJ). The second mass, measuring 6cms x 6cms, was 30cms from DJ. The third solid mesenteric mass measured 25cms x 20cms x 15cms and was 35cms from the DJ [Table/Fig-3]. The masses involved the mesenteric border of the jejunum but there was no infiltration into the jejunum. There were no enlarged lymph nodes, no ascites and both ovaries were normal.
Intraoperative finding of multiple jejunal masses (arrows)
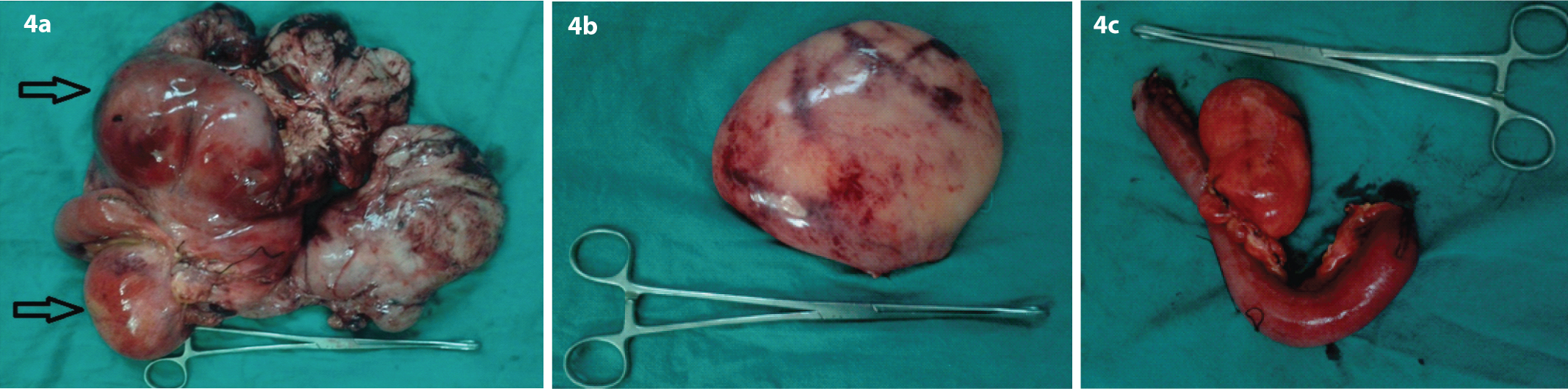
Complete excision of the mesenteric masses along with 1 foot of the involved jejunum and its mesentery followed by hand sewn double layer jejunojejunal anastomosis was done. The resected specimens are [Table/Fig-4].
(a) Resected gross specimen; (b) Largest resected mass; (c) Jejunal loop with proximal most mass
Patient recovery during the post operative period was uneventful and the patient was discharged on the 9th post operative day with a well healed surgical scar.
Based on the intra-operative finding of mesenteric origin of the masses, the provisional diagnosis of an ovarian tumour was ruled out. Similarly, the possibility of a retroperitoneal sarcoma or a peritoneal neoplasm was not considered. Masses originating from the mesentery viz. lipoma, liposarcoma and cysts had to be differentiated. This was not possible on gross examination. Therefore, the diagnosis was established conclusively on histopathology. The histopathological examination revealed the presence of a well differentiated liposarcoma with sudden transition to the dedifferentiated region. The presence of lipoblasts is essential for any diagnosis of liposarcoma [1]. The lipoblasts were distinctly large, irregular shaped with hyperchromatic nuclei in the well differentiated component. The dedifferentiated component was identified by the presence of multinucleated spindle cells. The histopathology report was consistent with the diagnosis of multiple dedifferentiated liposarcoma (R0 resection) of the jejunal mesentery with a Stage pT2b, Grade 2 tumour (Tumour differentiation score 3, Mitotic index score 1, Tumour necrosis score 0) as per FNCLCC (French Federation of Cancer Centres System Grading Scheme) [2]. It needs to be mentioned here that all the tumours had similar histopathological findings. Immunohistochemistry showed the dedifferentiated component to be negative for SMA, desmin, myoglobin, CD-34 and CD-117. However, the well differentiated region stained positive for S-100 protein. Fluorescence in situ hybridization (FISH) analysis demonstrated amplification of the MDM2 gene, which further cemented our diagnosis of Dedifferentiated Liposarcoma. [Table/Fig-5,6]
Histopathology sections of the tumour. A (HE stain 100x) Pleomorphic spindle cells interlaced with lipoblasts. B (HE stain 400x) Adipocytes with pleomorphic spindle cell having multinucleated and hyperchromatic nuclei. C (HE stain 100x) Transition zone between well differentiated and dedifferentiated component. D (HE stain 50x) Jejunum free from tumour

Immunohistochemistry of the tumour. A.(HE stain 100x) MDM2 overexpression in de-differentiated liposarcoma B. (HE stain 400x) MDM2 Positive lipoblast suggestive of de-differentiated liposarcoma . C. (HE stain 200x) S100 positive well differentiated component of the tumour. D. FISH image with Intranuclear red spots suggestive of amplification of MDM2 gene

Dedifferentiated mesenteric liposarcoma has a more aggressive clinical course than other types of high grade sarcomas and a high chance of recurrence [3]. Therefore, a decision to give adjuvant chemotherapy was taken. The patient, at the time of writing this paper, had received 6 cycles of Doxorubicin, Dacarbazine and Ifosfamide based adjuvant chemotherapy. The ultrasonography of the abdomen after the third and sixth cycle was normal. The patient was evaluated with a CECT abdomen after the sixth cycle and showed no recurrence of tumour. Follow up would consist of periodic evaluation and examination of the patient.
Discussion
A liposarcoma is the commonest soft tissue sarcoma that occurs in an adult, comprising 20% of all soft tissue sarcomas. It usually occurs in the deep soft tissues of extremities (75%) and retroperitoneum (20%) [4], however the mesentery being the tissue of origin is rare [5]. According to WHO, liposarcoma is classified into the following five categories: well- differentiated, pleomorphic, round cell, myxoid and dedifferentiated [6]. Dedifferentiated variety has a more aggressive course. A presentation with two synchronous mesenteric liposarcoma is very unusual [7]. A Pubmed search for mesenteric liposarcomas yielded 42 articles till date while searching with the keywords ‘Multiple primary mesenteric dedifferentiated liposarcoma’ yielded 8 results only, thereby making them a rare entity [8–15]. Liposarcomas usually occur during the 5th to 7th decades of life and the incidence is higher in males as compared to the females [16]. However, present patient was a 36 years old lady.
Our patient presented with dull aching pain and lump in the abdomen with associated constipation, loss of appetite and loss of weight. However, a patient may also present with an abdominal lump, low grade intermittent fever and weight loss without loss of appetite [17]. As is evident from this case, multiple mesenteric liposarcomas may present as a diagnostic dilemma for the surgeon, even after using the haematological, biochemical and radiological armamentarium available at hand. Intra-abdominal fatty masses which are likely to be malignant may show one or more of the following features on CT scan of the abdomen: inhomogeneity, infiltration or poor margination, CT numbers greater than the patient’s normal fat, or contrast enhancement [18].
It was only upon entering the abdominal cavity that the origin of the mass was discovered. In contrast to the CECT finding of a single mass arising from the pelvis, multiple masses originated from the jejunal mesentery. Surgical resection with negative margins (R0 resection) of mesenteric liposarcomas has a better probability of long-term survival of the patient [19].
For the present case, taking the radiological information and the intraoperative localisation of tumour into account, the possibility of Gastrointestinal Stromal Tumour (GIST) and sarcoma infiltrating fat can be considered. GIST characteristically stains with CD117 and does not consist of a Well differentiated liposarcoma (WDLPS) component. As proposed by Evans in 1979, Dedifferentiated liposarcomas (DDLPS) consist of a mixture of atypical lipoma/ Well differentiated liposarcoma and a high grade nonlipogenic sarcomatous component, with an abrupt transition between the two components [20] but the absence of well differentiated component does not rule it out, as this component can be scarce and therefore can be lost by sampling or simply not be present [21]. The prognosis of a patient corresponds to the histopathologic type of liposarcoma [20]. Dedifferentiated component usually appears like a high grade sarcoma and therefore, must be differentiated from malignant fibrous histiocytoma. The distinction between sarcoma infiltrating fat and dedifferentiated liposarcoma is made by the existence of the atypical lipomatous tumour alongwith well differentiated liposarcoma in the latter [3]. On Immunohistochemistry analysis, CD117 and CD34 do not stain the dedifferentiated region, however S-100 stains positively in the well differentiated region of the dedifferentiated liposarcoma [22]. Well-differentiated liposarcomas/atypical lipomatous tumours and dedifferentiated liposarcoma have amplification of several genes, including MDM2 [23,24]. Using immunohistochemistry, FISH, quantitative PCR, or comparative genomic hybridization (CGH) to identify MDM2 amplification aids in making an accurate diagnosis . Prognostically, 40% of the dedifferentiated liposarcomas will recur, 17% will metastasize and 28% will ultimately result in death of the patient [25]. Five cases of Dedifferentiated liposarcoma arising from the small bowel mesentery have been reported in literature [26].
The role of adjuvant chemotherapy for soft tissue sarcoma in adults was explored in a meta-analysis of 14 randomized trials. It concluded that there was significant improvement in the time to local and distant recurrence and overall recurrence free survival. However, no studies have been carried out for mesenteric liposarcomas [27]. The role of neo-adjuvant chemotherapy for mesenteric liposarcoma was evaluated in one study with Doxorubicin, Cisplatin and Ifosfamide. They reported that it was successful in shrinking a large ileocolonic mesenteric liposarcoma [28].
Conclusion
Present patient underwent complete surgical resection with six cycles of adjuvant chemotherapy and she has had no recurrence of tumour till present. Long term follow up and observation is required due to the high risk of recurrence of the dedifferentiated variety of liposarcoma.
[1]. Dei Tos AP, Pedeutour F, Atypical lipomatous tumor/well-differentiated liposarcoma. In Fletcher CDM, Unni K, Mertens F, editorsPathology and Genetics of Tumours of Soft Tissue and Bone, WHO Classification of Tumours 2002 LyonIARC Press:35-46. [Google Scholar]
[2]. Fletcher C.D.M, Unni K.K., Mertens F, Pathology and Genetics of Tumours of Soft Tissue and Bone. In: World Health Organization Classification of Tumours 2002 LyonIARC Press [Google Scholar]
[3]. Cha EJ, Dedifferentiated liposarcoma of the small bowel mesentery presenting as a submucosal massWorld J Gastrointest Oncol 2011 3(7):116-18. [Google Scholar]
[4]. Weiss SW, Rao VK, Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of “dedifferentiation”Am J Surg Pathol 1992 16:1051-58. [Google Scholar]
[5]. Son BS, Seok SJ, Chung SK, A case of liposarcoma arising in the mesenteryKorean J Gastroenterol 2009 54:243-7.doi:10.4166/kjg.2009.54.4.243 [Google Scholar]
[6]. DeVita VT, Lawrence TS, Rosenberg SA, DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology 2011 9th EdPhiladelphiaLippincott, Williams, Wilkins [Google Scholar]
[7]. Chan CC, Ghareeb E, A rare case of double mesenteric liposarcomaGr Round 2011 11:98-102.doi: 10.1102/1470-5206.2011.0024 [Google Scholar]
[8]. Ozguven S, Aras M, Inanir S, Mesenteric metastases of purely myxoid liposarcoma: An unusual behavior of primary tumor depicted on fludeoxyglucose positron emission tomography/computerized tomographyIndian J Nucl Med 2015 30(1):82-3.doi: 10.4103/0972-3919.147556 [Google Scholar]
[9]. Shen Z, Wang S, Fu L, Shi J, Yin M, Ye Y, Wang S, Therapeutic experience with primary liposarcoma from the sigmoid mesocolon accompanied with well-differentiated liposarcomas in the pelvisSurg Today 2014 Oct 44(10):1863-8.doi: 10.1007/s00595-014-0866-68 [Google Scholar]
[10]. Sachidananda S, Krishnan A, Ramesh R, Kuppurao S, Primary multiple mesenteric liposarcoma of the transverse mesocolonAnn Coloproctol 2013 Jun 29(3):123-5.doi: 10.3393/ac.2013.29.3.123 [Google Scholar]
[11]. Jukić Z, Brcić I, Zovak M, Vucić M, Mijić A, Kruslin B, Giant mixed-type liposarcoma of the mesentery: case reportActa Clin Croat 2012 Mar 51(1):97-101. [Google Scholar]
[12]. Korukluoglu B, Ergul E, Sisman IC, Yalcin S, Kusdemir A, Giant primary synchronously bilateral mesenteric dedifferentiated liposarcoma with hyperparathyroidism, hyperthyroidism, type-2 diabetes mellitus and hypertensionJ Pak Med Assoc 2009 Aug 59(8):563-65. [Google Scholar]
[13]. Remstein ED, Kurtin PJ, Nascimento AG, Sclerosing extramedullary hematopoietic tumor in chronic myeloproliferative disordersAm J Surg Pathol 2000 24(1):51-55. [Google Scholar]
[14]. Amato G, Martella A, Ferraraccio F, Di Martino N, Maffettone V, Landolfi V, Fei L, Del Genio A, Well differentiated “lipoma-like” liposarcoma of the sigmoid mesocolon and multiple lipomatosis of the rectosigmoid colon. Report of a caseHepatogastroenterology 1998 45(24):2151-56. [Google Scholar]
[15]. Nakamura A, Tanaka S, Takayama H, Sakamoto M, Ishii H, Kusano M, Onizuka Y, Ota S, Mitamura K, A mesenteric liposarcoma with production of granulocyte colony-stimulating factorIntern Med 1998 Oct 37(10):884-90. [Google Scholar]
[16]. Burgohain J, Kathiresan N, Satheesan B, Dumbbell-shaped mesenteric liposarcoma: A case report with review of the literatureThe Internet Journal of Surgery 2008 15 [Google Scholar]
[17]. Jain SK, Mitra A, Kaza RCM, Malagi S, Primary mesenteric liposarcoma: An unusual presentation of a rare conditionJ Gastrointest Oncol 2012 3(2):147-150.doi: 10.3978/j.issn.2078-6891.2011.051 [Google Scholar]
[18]. Friedman AC, Hartman DS, Sherman J, Lautin EM, Goldman M, Computed tomography of abdominal fatty massesRadiology 1981 139:415-29. [Google Scholar]
[19]. Heslin MJ, Lewis JJ, Nadler E, Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for managementJ Clin Oncol 1997 15:2832-39. [Google Scholar]
[20]. Evans HL, Liposarcoma: a study of 55 cases with a reassessment of its classificationAm J Surg Pathol 1979 3:507-523. [Google Scholar]
[21]. Coindre JM, Mariani O, Chibon F, Mairal A, De Saint Aubain, Somerhausen N, Favre-Guillevin E, Bui NB, Stoeckle E, Hostein I, Aurias A, Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytomaMod Pathol 2003 16(3):256-62. [Google Scholar]
[22]. Karaman A, Kabalar ME, Ozcan O, Koca T, Binici DN, Intraperitoneal dedifferentiated liposarcoma: a case reportWorld J Gastroenterol 2008 14:5927-29. [Google Scholar]
[23]. Karakousis CP, Dal Cin P, Turc-Carel C, Limon J, Sandberg AA, Chromosomal changes in soft tissue sarcomas. A new diagnostic parameterArch Surg 1987 122:1257-60. [Google Scholar]
[24]. Weaver J, Downs-Kelly E, Goldblum JR, Turner S, Kulkarni S, Tubbs RR, Rubin BP, Skacel M, Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasmsMod Pathol 2008 21:943-49. [Google Scholar]
[25]. Fletcher CDM, Unni KK, Mertens F, Pathology and Genetics of Tumours of Soft Tissue and Bone. In: World Health Organization Classification of Tumours 2002 LyonIARC Press:227-32. [Google Scholar]
[26]. Hasegawa T, Seki K, Hasegawa F, Matsuno Y, Shimodo T, Hirose T, Sano T, Hirohashi S, Dedifferentiated liposarcoma of retroperitoneum and mesentery: varied growth patterns and histological grades–a clinicopathologic study of 32 casesHum Pathol 2000 31:717-27. [Google Scholar]
[27]. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma adults: meta-analysis of individual data. Sarcoma Meta-analysis CollaborationLancet 1997 350:1647-54. [Google Scholar]
[28]. Ishiguro S, Yamamoto S, Chuman H, Moriya Y, A case of resected huge ileocolonic mesenteric liposarcoma which responded to pre-operative chemotherapy using doxorubicin, cisplatin and ifosfamideJpn J Clin Oncol 2006 36:735-38. [Google Scholar]