Quality assessment in Laboratory medicine is an essential requirement to ensure accuracy and precision of test results. Quality control programs with respect to Clinical Pathology and Haematology are being widely practiced world-wide. However, unlike the other branches of Laboratory Medicine, the scenario appears quite different with respect to implementation of quality control programs in the branch of histopathology. There are various factors attributing to its poor acceptance in histopathology, which includes cumbersome processing, subjectivity in reporting and lack of numerical data for easy assessment.
Histopathology laboratory comprises of various subunits and to assess quality, the entire processing has been categorized into pre-analytical, analytical and post-analytical phases. With respect to quality assessment in histopathology, we find that most studies available in literature are focused mainly on analytical diagnostic errors. However, pre-analytical and post-analytical phases should also be seriously assessed since these phases of test cycles are equally prone to errors [1].
The pre-analytical phase of tissue processing comprises of all the steps starting from receiving of tissue specimens until the submission of histopathology slides for interpretation. Incidence of errors occurring during tissue processing is also quite significant [2]. A uniqueness of pre-analytical phase is that it can influence the subsequent phases (analytical and post-analytical phases) thus making it a critical step. To the best of our knowledge there have been very limited studies documenting quality assessment in histopathology from India. These includes a review article on quality control in histopathology by Iyengar JN and a study by Agashe et al., focussing only on the analytical aspect in histopathology [3,4].
Pre-analytical phase serves as an important building block in evaluation of cellular pathology and delivery of accurate reports. However, most of the documented studies world-wide, focuses primarily on the analytical phase of quality evaluation in histopathology [1,5,6]. The present study was therefore initiated with the aim to evaluate and assess quality parameters in the pre-analytical phase in a histopathology laboratory.
Materials and Methods
This study was retrospective conducted at a histopathology laboratory at a tertiary health care centre in Chennai, India. Pre-analytical data spanning over a period of 34 months was analysed to assess seven quality variables: Registers, records and files were retrieved and checked and errors identified with respect to pre-analytical quality variables. The following parameters were studied-
Sample identification.
Specimen in appropriate fixative.
Lost specimen.
Sample rejection.
Daily quality control (QC) records of Haematoxylin & Eosin, special stain and immunostains.
Performance of the laboratory in inter-laboratory quality assessment programme for histopathology (ILQA- HP) by external quality assessment programme (EQAS).
Daily non-conformities (NC)
As per document available, Laboratory Director and Assistant Professor monitored quality measures in histopathology.
Sample identification: Manually labelled specimen containers bearing unique identification number received at the reception counter of histopathology matched with requisition form for patient identification. Registers were checked for agreement with respect to patient requisition form submitted and details on corresponding specimen container. Errors documentation with respect to any discrepancy were checked for and noted.
Specimen in appropriate fixative: For this histopathology lab all specimen were sent in container with 10% formalin to histopathology laboratory. Registers were checked to note for any incidence reported for specimen sent in inappropriate fixative or not in fixatives. Policy of this lab was to check for presence of appropriate fixative by the technician at reception counter. The fluid in specimen container was checked by taking small amount of the fluid and adding 1-2 drops of Schiff’s reagent to it. Development of pink colour indicated formalin, while no change in colour indicated presence of non-oxidizing fluid such as saline or water. Histopathology test in this information recorded in the nominal register was available for verifying any specimen not sent in formalin during the study period.
Lost specimen: Registers were checked for noting of any lost specimen during the study period.
Sample rejection: Registers were checked for any incidence of specimen rejection and reports of specimen returned to the Clinician/surgeon. However, in this hospital, the policy was not to reject any specimen received by the laboratory. The rule was to resolve any kind of discrepancy and accept the specimen.
Internal quality control performance: As a quality documentation process, the internal quality control performance with respect to Haematoxylin and Eosin (H&E), other histochemical stains and immunohistochemistry (IHC) were being assessed and scored daily as a part of routine work (Score 0: Unsatisfactory; Score 1:Poor; Score 2: Average; Score 3: Good; Score 4: Excellent). To assess quality of staining, the first slide from first batch was usually taken as the daily internal QC slide. In case of faulty staining in quality check slide, the probable cause was identified with rectification, and repeat standardization of staining method was done to prevent the subsequent batch from staining errors. Quality control slides of immunohistochemistry and histochemical stains were being filed daily. Documents of internal quality check available in the laboratory were perused to check for daily scores marked.
Performance in external/ inter-laboratory quality assessment: This laboratory participated in inter-laboratory quality assessment programme for histopathology (ILQA- HP) with respect to pre-analytical phase which was being conducted periodically (4 cycles/year) by another laboratory which was accredited for histopathology by National Accreditation Board for Testing and Calibration Laboratories (NABL). A tissue received in 10% formalin from nodal quality control centre was processed, sectioned and the stained slide was sent back to the nodal centre for pre-analytical quality assessment. The nodal centre on receiving the stained slides evaluated the quality and errors in processing, sectioning and staining. Parameters of processing, cutting and staining like thickness of section, artifacts and staining quality were scored by external assessing nodal centre. An analysis of performance was done for the study period.
Daily non conformities: Anything that deviates from normal and has a potential effect on the patient care is defined as non conformity (NC). Evaluation of daily non conformities was being maintained and registers were checked for identifying causes for NC during the study period.
This laboratory was well equipped with one automatic tissue processor, one slide warmer, three microtomes with disposable knives, three water baths, one wax bath and one autostainer, one semiautomatic immunostainer, two cryostats and one semiautomatic histokinette. Documents showed laboratory fluid (10% formalin, Xylene, wax and alcohol) in tissue processor was being changed based on number of tissue capsules processed (changes done after every 200 capsules) with downgrading of alcohol and daily internal QC performance. Blades for microtomes were changed after every 25 blocks. These were followed to ensure good quality tissue sections.
Results
A total of 18,626 tissue sample were processed in this histopathology laboratory from January 2007 to October 2009 (6600 in 2007; 6520 in 2008 and 5506 upto October 2009). This laboratory was accredited by National Accreditation Board for Testing and Calibration Laboratories (NABL). A quality check was done on specimen handling with respect to identification/labelling and submission in appropriate fixative [Table/Fig-1]. There was a low incidence of wrongly labelled specimens and specimen not sent in formalin [Table/Fig-1]. The recording showed immediate steps were taken to resolve the identity problem and specimens not sent in formalin were immediately put in 10% formalin.
Results of assessment on specimen details at reception counter.
| Year | Specimen wrongly labelled | Specimen notin fixative | Specimen lost | Sample rejection | Documentation | Steps taken to prevent it in future |
|---|
| 2007 | 3(0.04%) | 3(0.04%) | 0 | Nil | Done in all cases | Informed the in-charge nurse and clinician |
| 2008 | 1(0.01%) | 5(0.07%) | 0 | Nil | Done in all cases | Informed the in-charge nurse and clinician |
| 2009 | 1(0.01%) | 10(0.18%) | 0 | None cases | Done in all cases | Informed the in-charge nurse and clinician |
Sample rejection: No samples were rejected during the study period [Table/Fig-1]. The Policy of the laboratory was not to reject any sample, but in case of any discrepancy, documentation and rectification of the issue to be done before the specimen is submitted for further processing in histopathology laboratory.
Specimen not sent in fixative: Fixative routinely used was 10% formalin. Out of 18 specimens that were not sent in fixative, 14 were small biopsies and reached laboratory on the same day. They were immediately put in 10% formalin and showed no significant histological artifacts due to delay in fixation. Four were large specimens which included two placentas, one uterus and one tonsil. Tissue section of these showed significant fixation artifacts on histology but had no effect on outcome of the patients. Documentation was done in all faulty cases of specimen sent without fixative. Root cause analysis (RCA) revealed that frequent recruitment of new staff due to high attrition rate at the operation theater, was the possible cause for this error. Documents studied showed that in all these cases, the concerned ward/operation theatre nurse and treating clinician were informed. To prevent such occurrence in future orientation sessions for every new batch of recruits, and periodical re-education/re-inforcement regarding specimen labelling and use of appropriate fixative was taken as corrective and preventive measure (CAPA). This led to reduction of error of specimen in inappropriate fixative/non fixative. However, records showed that there was no documentation of quantity/volume of formalin in specimen containers (whether adequate or inadequate).
Lost specimen: There was no incidence of specimen lost in the entire study period [Table/Fig-1].
Internal quality control performance: The overall score analysis showed predominantly fair and good scores. Documents showed that whenever scores of below 3 was given, the probable reason for poor staining was looked for and steps were taken to improve the quality of next batch of slides.
Performance in External quality assurance program: Analysis of records for EQAS {ILQA HP program)} for pre-analytical phase showed satisfactory performance in 6 out of 9 cycles [Table/Fig-2]. The reason for low score performance was detailed by the ILQA laboratory. Two cycles showed errors related to staining and sectioning.
Performance score in inter-laboratory pre-analytical quality assurance programme
| Parameter assessed(Maximum score:5) | 2007 (3rd cycle) | 2007 (4th cycle) | 2008 (1st cycle) | 2008 (2nd cycle) | 2008 (3rd cycle) | 2008 (4th cycle) | 2009 (1st cycle | 2009 (2nd cycle) | 2009 (3rd cycle) |
|---|
| Processing (5) | 4 | 3.5 | 4.5 | 4 | 4 | 3 | 4 | 4 | @ |
| Sectioning (5) | 4 | 3 | 4.5 | 3.5 | 3.5 | *2.5 | 4.5 | *3 | 4/5 |
| H&E Staining (5) | 3.5 | *2.5 | 4.5 | 3.5 | 4 | 3 | 4 | 4 | 3/5 |
| Overall score | 11.5/15 | 9/15 | 13.5/15 | 11/15 | 11.5/15 | 8.5/15 | 12.5/15 | 11/15 | 7.5/10 |
Score 1: Unsatisfactory; score 2: poor; score 3; Average; score 4: Good; score 5: Excellent.
@: 3rd cycle of 2009 block was sent for only sectioning and PAS staining
*: Score <3 is advised to take immediate corrective action
Record showed that CAPA was done by our laboratory whenever a low score was obtained [Table/Fig-3].
| Score | Step | Error | RCA | RCA & CAPA |
|---|
| *2.5 | Staining | Haematoxylin weak | Defect in blueing | Standardize and improved blueing |
| *2.5 | Sectioning | Scores, tears scraped section | Faulty blades | Blade to be checked regularly for nicks and change after 25 blocks (earlier changed after 50 blocks) |
| *3 | Sectioning | Thick section | Human error | Discussed with technician advised to be more meticulous and check thickness more regularly |
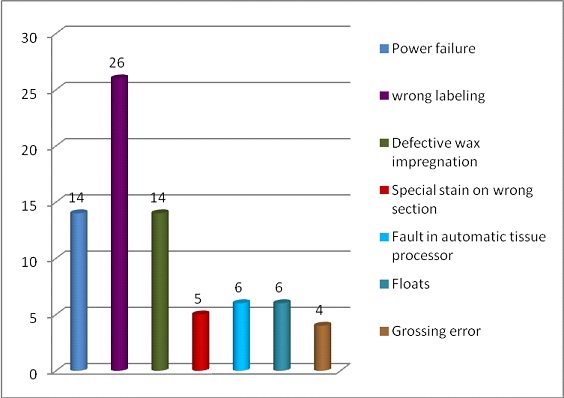
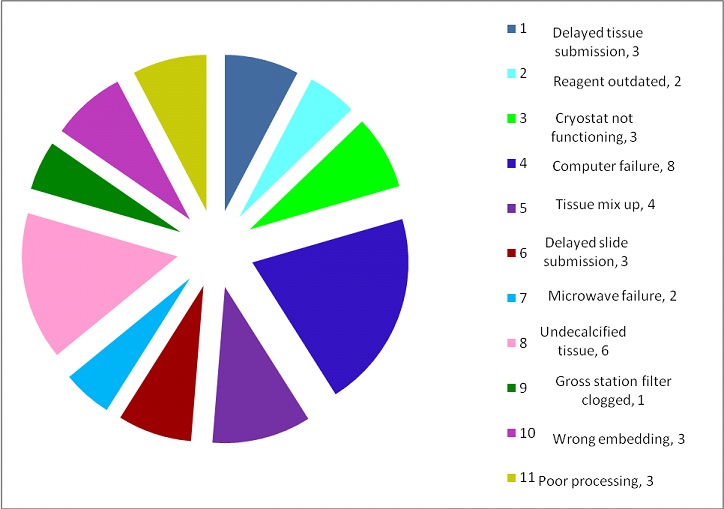
Daily non conformities: Daily NC record showed a variable trend and reason affecting the quality [Table/Fig-4,5 and 6]. Errors due to tissue processing noted in the non-conformity register accounted to 29 (14 cases with poor wax impregnation, 3 cases with poor dehydration and clearing, 6 cases with poor decalcification and 6 cases due to technical problem in tissue processor). Troubleshooting with Root cause analysis (RCA) and corrective and preventive action (CAPA) was performed in all the cases [Table/Fig-7].
Number of non-conformities identified in study period
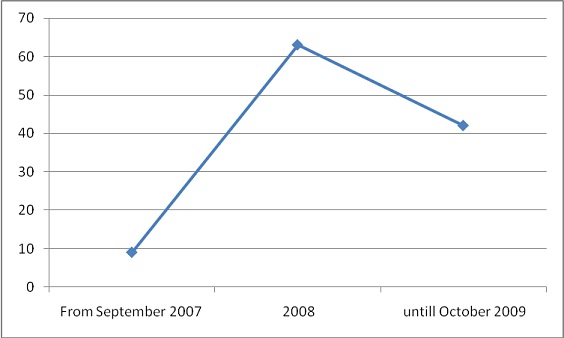
Non-conformities identified in 75/113 cases.
Other non-conformities identified in 38/113 cases.
Pre-analytical non conformities with root cause analysis and corrective and preventive action taken
| Non conformity | Root cause analysis | Corrective and preventive action |
|---|
| Power failure | No alternative power supply | Requested for UPS and successfully installed the UPS. |
| Wrong labelling | Illegible handwriting; human error | All technicians educated to check the label twice and its impact highlighted. |
| Defective wax impregnation | Automatic tissue processor technical fault; power failure | Engineer called for service and problem rectified with suggestion of regular service; UPS installed. |
| Special stain on wrong sections | Illegible handwriting | Pathologist, post graduates and technicians informed and consequences discussed. |
| Fault in automatic tissue processor | Technical fault | Alternative histokinette to be used; engineer called for service and suggestion of regular service. |
| Cryostat not functioning | Technical fault | Service help taken; older cryostat to be used as an alternative option. |
| Tissue mix up | Human error | Problem resolved with block identification; grossing technician informed and its impact highlighted. |
| Microwave failure | Technical fault | Department consensus to use pressure cooker for antigen retrieval step. |
| Un-decalcified tissue | Decal solution not working | Changed the solution from 10% to 20% formic acid in case of very hard bony specimens; Daily checks by grossing pathologist. |
| Gross station filter clogging | Technical | Engineer called for rectification; regular service suggested; To replace with better grossing station. Installed the new grossing station . |
| Floats | Inadequate knife washing, Floats picked in waterbath | Grossing resident, faculty and technical staff were educated about this. |
| Poor processing | Poor dehydration | Change of the 1st dehydration container with fresh absolute alcohol and downgrade the rest alcohol containers in tissue processor. |
Discussion
A well processed and good quality tissue section without any artifacts is the basic requirement for making an accurate histopathological diagnosis. A study by Meier et al., reveals that about 25% of all surgical pathology errors occur due to diagnostic misinterpretation, while incidence of wrong identification and defective specimens ranges from 27% to 38% and 4% to 10% respectively [7]. The fact that a significant number of errors occur during the pre-analytical phase, demands effective quality control and quality assurance steps during this phase of tissue processing [7].
Patient and specimen identification is the foremost essential step in this phase [5]. Wrong labelling of specimens has resulted in unwarranted procedures [5]. In case of any discordance, immediate communication with the clinician and concerned staff can resolve the problem of identity. Practice of bar coding system may be a useful step in reducing error of identification [1]. Root cause analysis (RCA) is a systematic process for detection of an error and its cause [2]. Only after RCA is done a corrective and preventive action (CAPA) can be taken and implemented to avoid its occurrence in future. The present laboratory, with its adherence to this policy follows patient name and a unique hospital number as alphanumeric identifiers and showed a very minimal error with respect to wrong labelling, which were identified and documented with immediate RCA and implementation of CAPA.
Histopathology laboratory is quite different from a chemical/Haematology laboratory with respect to sample collection. In many instances histopathology laboratory receives totally excised lesion. In image-guided biopsy, subjecting the patient for a re-biopsy can be a tedious process. Hence, policy of sample rejection cannot be valid for histopathology.
Incidence of lost specimen is another key element in pre-analytical phase [8]. There was no incidence of lost specimens during the period of our study, thereby reflecting a thorough system of specimen transfer from operative theatre/ward to histopathology laboratory. Lost specimen would require repeat sampling causing unnecessary delay in diagnosis and treatment.
Clinical history may influence the accuracy and completeness of reports [9]. An adequately filled clinician request form is an essential tool to guide the pathologist, which directly influences the overall turnaround time (as additional time would be spent in contacting the clinician for obtaining the required information). Availability of patient’s information facilitates the histopathologist to narrow down the differential diagnosis. Previously published studies on clinician’s request form for histopathology showed a variable data. Sharif et al., found absolutely no clinical details in 34% of their cases [10]. In contrast to this, in similar focused studies, Nakleh et al., and Burton et al., found a lower incidence of inadequate clinical details in their study with 2.4% and 6.1% respectively [8,11]. Varying level of sensitization of the clinicians, to importance of providing salient clinical details in request forms maybe a factor for such contrasting results in above studies. In this study we did not peruse requisition forms for evaluating this aspect.
Fixation is the key step that not only affects histological sections but also antigen retrieval for immunohistochemistry [10,12,13]. Poor fixation would result in poor morphology due to autolytic changes, thereby limiting proper histopathological interpretation and diagnosis [10,14]. Sending the specimen in inappropriate fixative (especially in a tropical country like India) can have an adverse effect on specimens, as the tissue undergoes faster autolysis due to high atmospheric temperature. An inadequate report generated out of poor fixation, in many circumstances would put the clinician in dilemma with regards to further treatment (in case of the lesion that has been completely excised and sent for histopathological examination). In our RCA, we found that the most commonly misused material as fixative was saline or tap water instead of 10% buffered formalin.
Grossing of specimen forms an important part of pre-analytical phase. In case of large specimen marking out the bitting area can be a useful step to identify the exact location of the sample tissue studied and can also be of immense help in re-bitting. Incidence of re-bitting could be an indicator to assess quality of grossing of specimens. Daily running of controls for routine/special stains as a regular procedure is a highly recommended quality assessment and improvement step. This as a quality step is practiced to ensure satisfactory sectioning and staining thus enabling the pathologist in clear identification of cellular morphology [3]. A well stained section is one of the hallmark of a good and proper functioning histopathology laboratory.
There was no special recording of sectioning defects in internal quality control file. Under-processed tissue, poor sectioning and faulty cutting would result in unnecessary re-works. Adhering to strict criteria by the laboratory for chemical change in processing and number of blocks to be cut with each blade would help in bringing out quality sections.
The laboratory studied participated in pre-analytical external quality assurance programme in which tissue was received in 10% formalin. This unbiased opinion from an external quality assessment programme in the form of scores from the nodal centre helps in evaluating and correcting oneself. Performance in such programme is one of the important pre-analytical quality indicator of a laboratory and is essential for quality assessment and improvement.
The aim of filling up of daily NC register is to investigate the irregularities and identify the cause and implement CAPA and prevent such occurrences in future. The daily NC register was evaluated and about 92.9% of cases identified belonged to pre-analytical phase. The non-conformance analysis in the present study showed four cases had extraneous material in slides. Presence of floats can lead to an erroneous diagnosis [15]. Availability of clinical history and an observant pathologist can easily identify such material avoiding a wrong diagnosis. Technical staff and grossing pathologist, mainly postgraduate pathology students/residents should know the steps that can bring extraneous material. First is the pick-up during grossing (contaminated work station, blades, knifes, forceps), second is at the time of paraffin embedding (contaminated embedding forceps) and the last is floaters from the water bath (poorly cleaned water bath). Steps 1 & 2, the re-cuts will also show the extraneous material as they are embedded in the paraffin block. However, it will not be seen in the re-cuts if it is has been transferred onto the slide (floaters) while picking up sections from the water bath. In cases reported as floats in this lab, a root cause analysis was done on above lines and confusion resolved. Thorough washing and cleaning of the work station, blade, knife, forceps and other instruments and the embedding forceps at the time of paraffin embedding will avoid the contamination. Effective cleaning of water bath has to be stressed to the technical staff to avoid contamination.
In our study we found wrong labelling of slides as one of the most frequent error. Morelli et al., in a similar study also identified this as the commonest error in pre-analytical phase and they attributed this error to lack of automation in numbering of specimen containers [2]. Daily NC file evaluation also revealed that there were instances when special stain was done on wrong sections resulting in delay in final opinion. Root cause analysis showed that it was either due to illegible hand written instruction by pathologist or visual error by technician. Mislabelling noted in our cases re-emphasizes utilization of bar coding technology in the laboratory.
Troubleshooting for various problems and errors encountered were observed as a part of daily quality monitoring (Root cause analysis) and preventive and corrective actions were taken (as mentioned in [Table/Fig-7]). Processing defects such as poor wax impregnation, tissue mix ups, poor decalcifications, floats and poor dehydration were pre-analytical errors identified during regular monitoring of quality. Under-processed tissue and faulty cutting would result in unnecessary re-works. Adhering to strict criteria by the laboratory for chemical change in processing and number of blocks to be cut with each blade would help in bringing out quality sections. In the present lab fluid in tissue processing was being changed and alcohol downgraded based on tissue blocks processed (change every 200 blocks) which assured quality in tissue processing. Scores, tears, thick sections were pre-analytical errors reported in interlaboratory quality assurance programme (EQAS programme) which made lab to take decision of changing microtome blades after cutting 25 blocks ([Table/Fig-3] shows troubleshooting). Power problem resulted in defective wax impregnation affecting cutting of blocks by microtome and poor quality of tissue sections. Although fault in tissue processor did affect quality of dehydration, clearing and wax impregnation, but did not overall affect the turnaround time (TAT) due to availability of an alternative histokinetic. Hence, a backup instrument in histopathology laboratory is essential to overcome any breakdown. To ensure good quality standards it is very important to identify mistakes and prevent its occurrence in future by initiating preventive actions. Discussion with technicians involved, Pathology residents/Pathologists about the errors is one of the key steps to ensure practice of preventive steps in the labs and this study highlights this fact.
A laboratory intending better quality performance must encourage each individual working in the laboratory to raise a NC. In initial phase of any laboratory implementing quality control procedures one may find more number of NC. However, with rectification of problems, one may find a down trend in incidence of NC. Daily NC evaluation in this laboratory showed a varied trend of problems which were investigated and steps were taken to resolve the problem. Sometimes, extra NC maybe identified in the laboratory due to more sensitization. One point to be emphasized to all the staff in the lab is that “it is a fact finding and not fault finding mission” and strictly, no punitive action should be taken based on NCs raised.
Limitations
Pre-analytical phase is quite an extensive phase in histopathology processing. Hence, analysis of clinician requisition forms and grossing errors could not be included in this study. A more focused study in future on these parameters can add useful information.
Conclusion
A satisfactory level of quality was being maintained in the histopathology laboratory studied with respect to pre-analytical phase, with a scope for further improvement. A proper RCA and application of CAPA reduces incidences of errors and thereby improves the quality of health care delivery system. Since, pre-analytical phase includes various crucial steps that would affect the interpretation, hence it becomes important for implementing a defined quality system for surveillance of errors and mistakes.
Score 1: Unsatisfactory; score 2: poor; score 3; Average; score 4: Good; score 5: Excellent.
@: 3rd cycle of 2009 block was sent for only sectioning and PAS staining
*: Score <3 is advised to take immediate corrective action