Epilepsy occurs in 0.5 to 1% of the population and begins in childhood in 60% of the cases [1]. Incidence peaks in the first year of life. A number of antiepileptic drugs (AEDs) have been used in seizure control. Treatment is to be initiated with a single drug, which should be least toxic, easy to handle, acceptable and economically affordable. The goal in every patient should be the use of only one drug with the fewest possible side effects for the control of seizures. The drug is increased slowly till the seizure control is accomplished or until undesirable side effects appear [2]. Because epilepsy involves a prolonged treatment, a majority of the times, the concern about toxicity, becomes the most important factor in the selection of a drug [1].
Gingival overgrowth has been recognized since long as a deleterious effect of chronic phenytoin therapy. It has also been reported with the use of sodium valproate, carbamazepine and phenobarbitone [3,4]. Many studies have been carried out on drug induced gingival overgrowth but still there are many misunderstood and inexplicable aspects of this condition. Kimball in 1939 was the first to report this peculiar side effect on gingival tissues caused by phenytoin [5]. Since then, other agents have been introduced that have also been linked to clinically significant forms of gingival enlargement. For example gingival enlargement cases after chronic use of valproic acid, carbamazepine, or phenobarbitone have been reported. However, they have been poorly documented. Vigabatrin, a new AED has recently been implicated for causing gingival overgrowth [6].
It has been observed that not all patients exhibit gingival overgrowth. However, most authors estimate the incidence of phenytoin induced gingival overgrowth to be approximately 40 to 50% [5,7–9].
Furthermore, considerable controversy exists as to the part played by certain local and systemic factors. Published literature suggests a correlation between oral hygiene, gingival inflammation and periodontal probing depth but the techniques used for establishing these relations have been varied and there exist a multitude of variables affecting this correlation [10–12].
Phenytoin, carbamazepine and sodium valproate are among the more commonly prescribed first line of drugs for the control of epilepsy [13,14].
The aim of the study was to identify the antiepileptic drugs (phenytoin, carbamazepine and sodium valproate) that have an effect on gingival overgrowth and to determine the factors effecting gingival overgrowth following antiepileptic drug (AED) therapy.
Materials and Methods
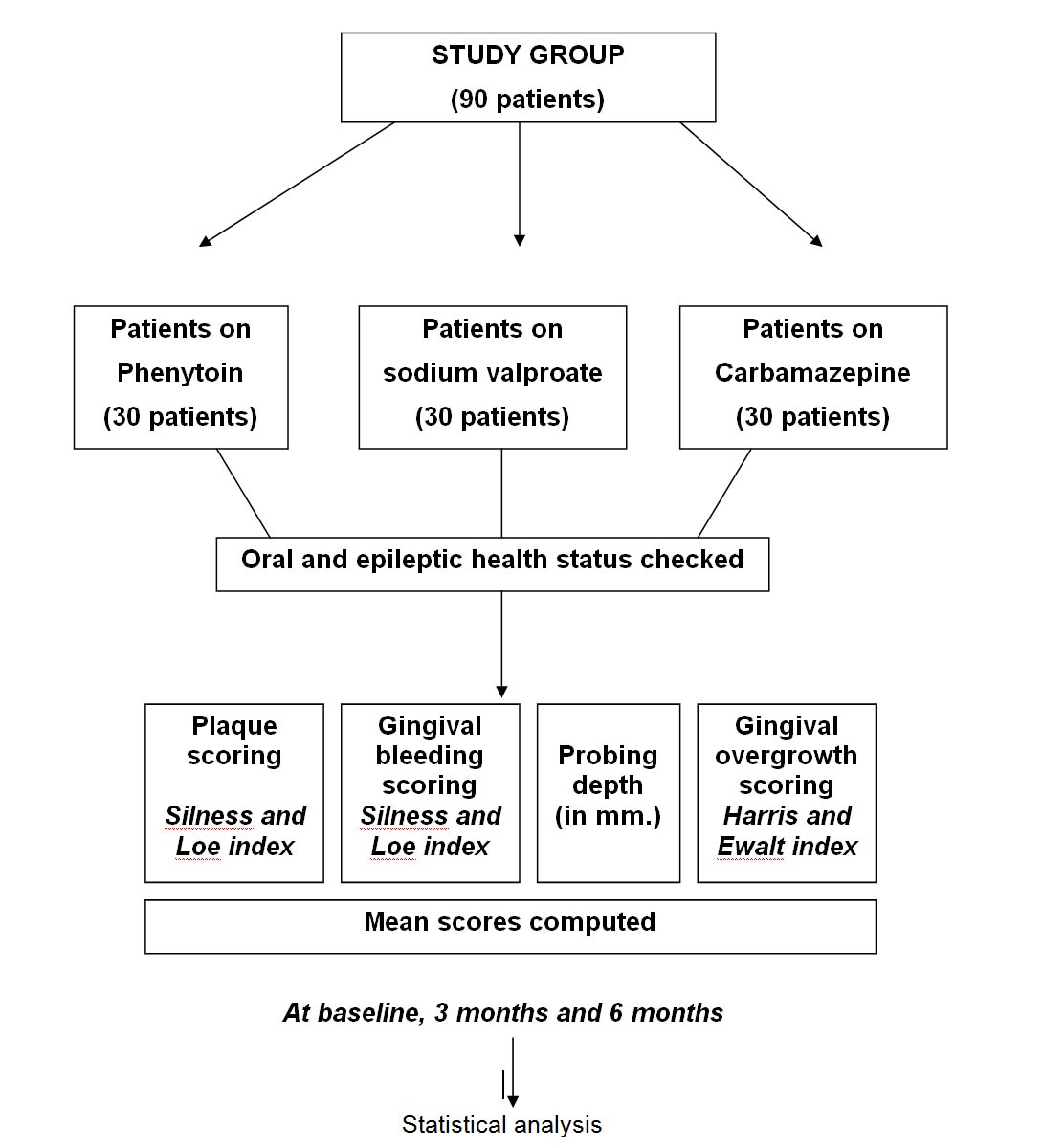
The present study was carried out in the Department of Pedodontics and Preventive Dentistry, Christian Dental College, Ludhiana and the Department of Neurology, Christian Medical College, Ludhiana. Patients (age 8-18 years) with epilepsy and on AED’s attending the Neurology out patient Department were the study subjects. The study was conducted on 90 children on AED’s. Thirty patients per drug (phenytoin, sodium valproate, carbamazepine) were checked and were grouped as below:
Group I - patients on phenytoin,
Group II - patients on sodium valproate,
Group III - Patients on carbamazepine.
Inclusion Criteria
Recently diagnosed cases of epilepsy, on AEDs for a maximum period of six months.
Patients with new onset epilepsy, initiated on AEDs.
After the informed consent of the parents/guardians, the patient’s were assessed as follows:
(1) Assessment of the general dental health status. A detailed history and examination of the patient’s oral health status was recorded.
(2) Assessment of the patient’s epileptic condition and the AED therapy status. The hospital records were reviewed: the age of onset, the type of seizure, the frequency of seizure, duration of epilepsy were recorded.
(3) Assessment of the gingival, periodontal health status, and its reevaluation during the period of study. This included recording of the dental plaque score (Silness and Loe [15] Index on all the teeth), gingival bleeding score (Loe and Silness Index [16] on all the teeth), probing depth (Willams graduated periodontal probe, facially, lingually as well as proximally for each tooth, and the degree of gingival overgrowth (as per the modified Harris Ewalt index 1942) [17,18]. The oral health measures taken by the patient were also recorded.
The patients were examined at baseline, at the end of three months and finally at the end of six months. All the findings (change in scores of the mentioned indices and probing depth) were recorded and put to statistical analysis. [Table/Fig-1] describes in brief the methodology used in the study.
Flow chart describing the methodology.
Assessment of gingival overgrowth in every drug group.
Correlation of gingival overgrowth with plaque scores, gingival bleeding scores, probing depth and epileptic and oral health findings
Results
The overall mean age of the sample was 13.16±2.44 years. There were 51 males (56.7%) and 39 females (43.3%) in the sample.
Gingival overgrowth by the end of 6 months, 16 out of 30 patients on phenytoin showed some degree of gingival overgrowth. Two out of 30 patients on sodium valproate showed minimal degree of mean gingivaI over growth. None of the patients on carbamazepine showed any evidence of gingival overgrowth. The mean values are described in [Table/Fig-2]. In Group I, there was a significant difference in the means when compared at baseline and 3 months and baseline and 6 months (p<0.05). There was no significant difference in between 3 months and 6 months (p>0.05). In Group II, there was an insignificant difference in the means when compared at baseline and 3 months and baseline and 6 months; (p>0.05). There was no difference in the means of gingival overgrowth when compared between 3 months and 6 months. Group Ill had not shown any gingival overgrowth [Table/Fig-3].
Mean gingival overgrowth at baseline and follow up.
| Follow up | At baseline | At 3 months | At 6 months |
|---|
| Mean ± SD | Mean ± SD | Mean ± SD |
|---|
| Group I | 0.08 | 0.20 | 0.32 | 0.44 | 0.35 | 0.48 |
| Group II | 0 | 0 | 0.01 | 0.04 | 0.01 | 0.04 |
| Group III | 0 | 0 | 0 | 0 | 0 | 0 |
Comparison of means of gingival overgrowth at different time intervals., *Significant
| Time period | Groups |
|---|
| I | II | III |
|---|
| t | p | t | p | t | p |
|---|
| Base line vs 3 months | 2.74* | <0.05 | 1.38 | >0.05 | -- | -- |
| Base line vs 6 months | 2.87* | <0.05 | 1.38 | >0.05 | -- | -- |
| 3 months vs 6 months | 0.25 | >0.05 | -- | -- | -- | -- |
[Table/Fig-4] describes distribution of cases according to the severity of mean gingival overgrowth. Mean gingival overgrowth at 6 month was seen more on the buccal side as compared to the lingual side [Table/Fig-5] and was seen to be maximum in the lower anterior buccal sextant. It was considerably less in the posterior sextants [Table/Fig-6].
Distribution of cases according to severity.
| Severity | No. of cases | Percentage |
|---|
| Minimal | 11/16 | 68.75% |
| Moderate | 5/16 | 31.25% |
| Marked | 0 | 0.00 |
| Severe | 0 | 0.00 |
Buccal side vs. Lingual side.
| Side | GO at 6 months |
|---|
| Upper Lingual | 0.57±0.62 |
| Upper Buccal | 0.69±0.69 |
| Lower Lingual | 0.63±0.69 |
| Lower Buccal | 0.78±0.76 |
Mean gingival overgrowth at 6-months, per sextant *.
| Sextant | | GO at 6 months |
|---|
| 1 | Lingual | 0.27±0.39 |
| Buccal | 0.41±0.48 |
| 2 | Lingual | 1.14±0.62 |
| Buccal | 1.29±0.69 |
| 3 | Lingual | 0.29±0.39 |
| Buccal | 0.38±0.47 |
| 4 | Lingual | 0.31±0.44 |
| Buccal | 0.42±0.47 |
| 5 | Lingual | 1.28±0.64 |
| Buccal | 1.49±0.74 |
| 6 | Lingual | 0.31±0.46 |
| Buccal | 0.43±0.46 |
*1=right upper posterior, 2= upper anterior, 3=left upper posterior, 4= leftlower posterior, 5 =lower anterior, 6 = right lower posterior
Plaque Scores
There was no significant difference between the mean plaque scores when they were compared at baseline, 3 months and 6 months in all the 3 drug groups [Table/Fig-7]. To check for any correlation between plaque scores and gingival overgrowth, correlation coefficient was computed for plaque index and gingival overgrowth at the baseline, three months and 6 months and the values were 0.154, 0.327 and 0.341 with none of the values being significant (p>0 .05)
Mean Plaque scores in the 3 drug groups.
| Follow up | At baseline | At 3 months | At 6 months |
|---|
| Mean ± SD | Mean ± SD | Mean ± SD |
|---|
| Group I | 1.43 | 0.66 | 1.53 | 1.23 | 1.53 | 0.73 |
| Group II | 1.23 | 0.66 | 1.23 | 0.05 | 1.25 | 0.66 |
| Group III | 1.43 | 0.56 | 1.42 | 0.58 | 1.48 | 0.59 |
Gingival Bleeding Scores
There was no significant difference between the mean gingival bleeding scores when they were compared at baseline, 3 months and 6 months [Table/Fig-8]. To cheek for any relation between presence of gingivitis and gingival overgrowth, correlation coefficient was computed between gingival bleeding and gingival overgrowth at baseline, at 3 months and 6 months. The values were 0.094, 0.208, 0.230 respectively, none of them being significant (p>0.05).
Mean gingival bleeding scores in 3 drug groups.
| Follow up | At baseline | At 3 months | At 6 months |
|---|
| Mean ± SD | Mean ± SD | Mean ± SD |
|---|
| Group I | 0.83 | 0.34 | 0.90 | 0.44 | 0.92 | 0.40 |
| Group II | 0.71 | 0.50 | 0.77 | 0.51 | 0.78 | 0.71 |
| Group III | 0.67 | 0.34 | 0.70 | 0.34 | 0.72 | 0.35 |
Probing Depth
The mean probing depth (in mm) in the 3 drug groups at baseline and follow up is described in [Table/Fig-9]. To check for any relation between probing depth and gingival overgrowth, correlation coefficient was computed for the amount of probing depth and gingival overgrowth at baseline, 3 and 6 months period, the values being 0.129, 0.337and 0.364 respectively. A significant relation (p<O.05) between probing depth and gingival overgrowth was seen at 6 months
Mean probing depth (in mm.) in 3 drug groups.
| Follow up | At baseline | At 3 months | At 6 months |
|---|
| Mean ± SD | Mean ± SD | Mean ± SD |
|---|
| Group I | 2.89 | 0.81 | 3.07 | 0.97 | 3.13 | 0.97 |
| Group II | 2.60 | 0.44 | 2.62 | 0.44 | 2.63 | 0.44 |
| Group III | 2.51 | 0.40 | 2.57 | 0.39 | 2.50 | 0.40 |
The epileptic and oral health status of each patient was assessed at baseline and the findings recorded. No correlation could be established between gingival overgrowth and any of the epileptic factors, irregularity of teeth, frequency of brushing or severity of dental caries at the end of this study.
Discussion
The fact that phenytoin causes gingival overgrowth is well known. But whether sodium valproate has a similar effect on gingiva is not clear in the published literature. A few case reports have been published. One was about a 15-month-old male [19]. Gingival overgrowth was seen one month after the initiation of drug therapy. In another reported case gingival overgrowth occurred in a 14-year-old female, 18 months after the initiation of therapy [20]. In yet another case reported by Anderson et al., the tissue resembled gingival overgrowth caused by other drugs in both clinical and histological appearance [21]. A case of a neonate with fetal valproate syndrome was reported Banita et al., [22]. Joshipura has reported an interesting case in which the patient did not develop the enlargement in spite of taking phenytoin for one year and developed enlargement with sodium valproate within 6 months [23].
But no follow-up studies starting from beginning of drug therapy have been reported. Seymour et al., in a study on adult epileptics, concluded that sodium valproate was a safe drug as far as gingival overgrowth was concerned and could be considered an alternative to phenytoin [24]. The present study draws a conclusion that sodium valproate may result in gingival overgrowth in children although to statistically insignificant levels in six months. It is recommended that studies be carried out on patients taking sodium valproate to check its effect on the gingiva over a longer period of time.
Carbamezapine has not been directly implicated in gingival overgrowth, but a few studies ambiguously mention it amongst the drugs resulting in gingival overgrowth [4,25]. In a previous study, published in 1996 periodontal condition was studied in 8-18-year-old patients who had been undergoing treatment with phenytoin, carbamazepine or sodium valproate [26]. Gingival enlargement was found in 30% of the patients but no correlation with the AED was done. From the results of the present study, it can be concluded that carbamazepine does not have an effect on gingival overgrowth.
It can be deduced that gingival overgrowth occurs in the first few months of phenytoin drug therapy. This is in accordance with the previously published studies [27]. O’Neil and Figures and have reported significant gingival enlargement to occur even earlier that is one month after the commencement of medication [28].
The incidence in this sample was found to be 53.6%. In the published literature, the frequency of drug induced gingival overgrowth ranges from very low percentages to 95% among the phenytoin users. This variability, apart from the lack of uniform criteria for grading, presence or absence of gingival overgrowth and disparate background of the examiners could be due to differences in methodology regarding case selection procedures. In this study, out of the total affected patients, 68.75% manifested signs of only minimal mean gingival overgrowth while the 38.75% manifested moderate mean gingival overgrowth. Previous studies have shown similar results in this regard. Those studies, which tended to give a prevalence of around 50%, had a high representation of low-grade gingival overgrowth [29].
Amongst the patients, who manifested gingival overgrowth, the gingiva of the buccal side was found to be affected to a greater extent and with greater severity than the gingiva of lingual side. Gingival overgrowth also manifested with greater extent and severity in the anterior region especially the lower anterior sextant. These findings are despite the known fact that oral hygiene is generally always better in the anterior region. A plausible explanation for such site specificity has till date evaded various workers all over the world.
The role of plaque and gingival inflammation has been controversial in literature. Dental plaque as a cofactor in the etiology of gingival overgrowth has been mentioned in the periodontal diseases classification system wherein drug induced gingival diseases are categorized into the major group ‘dental plaque induced gingival diseases [30]. Whereby, not only the severity but also development was pointed as being influenced by accumulation of dental plaque. It has been postulated that presence of dental plaque may provide a reservoir for the accumulation of the drug thus posing a risk factor for gingival overgrowth [31]. Dental plaque affecting gingival overgrowth has been shown in a number of studies [11,32]. On the contrary, some other writers have reported no correlation between gingival overgrowth and poor oral hygiene [33,34]. A genetic link has also been previously given as an explanation as to why some people even with poor oral hygiene do not develop gingival overgrowth [35,36]. A recent publication describes that there exists a complex interplay of various factors viz. fibroblast biology, connective tissue turnover, growth and inflammatory processes in a larger backdrop of genetic susceptibility [37].
The concept that gingival dental plaque predisposes to gingival overgrowth could not be established in this study. In this study, patients showing similar degree of oral hygiene have shown vastly different degrees of gingival overgrowth. It can be concluded from the present study that basic overgrowth tendency of the gingiva due to an AED is not influenced by the presence of plaque.
The concept that gingival inflammation (as measured by gingival bleeding score) predisposes to gingival overgrowth also could not be established in this study. This is in contrast to the findings of Aas, Angelopoulos, King et al., [35,37,38]. Gupta et al., had stated in their study that phenytoin had an anti inflammatory effect [39]. Inflammatory cytokines (IL 1 B and TNF A) released in the cases of gingival inflammation have also been implicated in the pathogenesis of gingival overgrowth [40]. Glickman and Lewitus have stated that the oedema which results from gingival inflammation is capable of influencing the hyperplasia, but the nature of the influence is not related to the regulation or initiation of hyperplastic tendency [41]. Other investigators have suggested that inflammation is a complicating factor rather than an initiating factor [42]. It can be concluded from the present study that gingival inflammation and oral hygiene are so closely associated that findings on gingival inflammation may be regarded as parrelling the state of oral hygiene or at least its therapeutic effectiveness and that the basic hyperplastic tendency of the gingiva due to an AED is not influenced by its inflammatory condition.
Depth of periodontal pocket on probing is recognized as a potential risk factor for all drug induced gingival overgrowth [4]. But, increase in probing depth can also be due to the increased bulk of gingiva [43]. Therefore, although probing depth can be correlated with gingival overgrowth, such a deduction needs to be done with caution because increase in probing depth can also mean the vertical manifestation of increase in gingival overgrowth. Checking for the loss of attachment was not included in the methodology, which can be considered to be a limitation of the study.
It is recommended that similar studies be carried out on other AED’s. Since the beginning of the last decade, newer AED’s have been introduced [44]. This has been spurred by the fact that the available AED’s did not provide optimal care for the patient with epilepsy. These new AED’s are not known as yet to have toxic side effects like osteoporosis, alteration in endocrine function or gingival overgrowth [45]. Although, vigabatrin, a new AED has recently been implicated for causing gingival overgrowth [46].
Evidence does exist, either from comparative or dose controlled trials that gabapentin, lamotrigine, topiramate and oxcarbazepine have efficacy as monotherapy [45]. It is recommended that more studies be designed to elicit the effect of the new AED’s on the gingiva and the periodontium and subsequently, substitution by the newer AED be recommended if they are found effective and safe.
Conclusion
Carbamazepine can be considered a safe drug in children in relation to gingival overgrowth. Sodium valproate has the potential to cause gingival overgrowth in children. In the present study, it led to gingival overgrowth, but not up to statistically significant levels. It is recommended that studies be carried out on patients taking sodium valproate to check its effect on the gingiva over a longer period of time. Phenytoin can cause gingival overgrowth in a significant number of children. In this study the incidence was reported to be 53.6%.
Plaque levels and gingival inflammation do not affect the initiation of gingival overgrowth. Probing depth could be positively correlated with gingival overgrowth in this study. Substitution of the AED causing gingival overgrowth is a viable alternative. Similar studies need to be done on newer AED’s to check for their effect on gingival overgrowth.
*1=right upper posterior, 2= upper anterior, 3=left upper posterior, 4= leftlower posterior, 5 =lower anterior, 6 = right lower posterior