Introduction
Dietary habits can make a big difference on both physical and mental aspects of the body. Menstrual disorder frequently affects the quality of life of adolescent and young adult women. Menstrual cycle irregularities may be associated with psychological stress, and endocrine disturbances. Monitoring of sensory-motor association and cardiovascular activity across the menstrual cycle has not been evaluated with dietary habits.
Aim
The present study was carried out to bridge the relationship between dietary habits and endogenous sex hormone mediated sensory motor association and heart rate variability (HRV) among young females during different phases of menstrual cycle.
Materials and Methods
The present study was carried out on healthy volunteered 100 female medical students in the age group of 19-25 years with regular menstrual cycle. Group I (n=45) vegetarians, Group II (n=25) eggetarians and Group III (n= 30) non-vegetarians, where n denotes the number of individuals in each group. Sensory-motor association (reaction time) and cardiovascular activity (HRV) was evaluated.
Results
We observed among all the dietary habits (vegetarians, eggetarians and non-vegetarians) the reaction time and HRV was comparable in follicular and menstrual phase, however it was significantly altered in luteal phase when compared to follicular and menstrual phase. Moreover, among all the dietary habits, non-vegetarians showed more significant alteration of reaction time and HRV in luteal phase when compared to vegetarians and eggetarians, as well as there was positive correlation between visual and auditory reaction time and negative correlation between LF and HF in luteal phase, among all the dietary habits.
Conclusion
We concluded sensorimotor association and regulation of autonomic tone is modified in luteal phase comparable to follicular phase and menstrual phase; however non-vegetarian had showed more significant alterations as compared to eggetarians and vegetarians. These suggest that sympathetic nervous activities are predominant in the luteal phase as compared to follicular phase, and this sympathetic dominance is more among non-vegetarians, which may be due to their higher BMI. The alterations in the balance of ovarian hormones might be responsible for these changes. Long-term intake of vegetarian diets may facilitate vagal regulation of the heart without increasing the sympathetic modulations of the cardiovascular system.
Introduction
Dietary habits are important factors that affect human life styles. Dietary habits in young adult women must be assessed from the awareness of total assistance throughout whole life [1]. Food intake and selection is influenced by neurochemical, hormonal, physiological and psychological factors. Dietary habits may significantly influence menstrual function in young women. The menstrual cycle (28- to 29-day cycle) may be divided into two general phases, the follicular and luteal phases [2]. The follicular or proliferative phase extends from Day-1 (the first day of menstruation) to Day-14 and luteal or secretory phase generally extends from Days 14 to 28. Low serum concentrations of estrogen and progesterone characterize the early follicular phase. Estradiol peaks occur in the pre-ovulatory surge just prior to ovulation, though progesterone levels remain low. The follicular phase ends at the time of ovulation. The high concentrations, of both estrogen and progesterone characterize the luteal or secretory phase. Cycle phase can be estimated by counting backward to the first day of menstruation. Natural fluctuations in ovarian hormones across the menstrual cycle allow for noninvasive studies of the effects of endogenous hormone (estrogen, progesterone, luteinizing hormone and follicle stimulating hormone) on cognition impairment and heart rate variability in young females. HRV is a non-invasive method to assess cardiac autonomic control system. Heart rate variability (HRV) finds out the relative influence of the sympathetic and parasympathetic of the autonomic nervous system (ANS) of the heart and therefore serves as an index and measurement of ANS activity. Previous studies have assessed sensory motor association and HRV across the menstrual cycle in healthy young adult [3,4] but still it has not been correlated along with dietary habits. There is increasing interest in the role of sex steroid hormones on sensory motor association and HRV. Two main frequency components of HRV, low frequency (LF, 0.04–0.15 Hz) reflecting the interaction of both sympathetic and parasympathetic (vagal) nervous systems, and high frequency (HF, > 0.15 Hz) reflecting the activity of the parasympathetic nervous system [5]. In female, HRV get influenced by endogenous sex hormones [6] and menstrual cycle [7]. Reaction time (sensory motor association) is the time interval between the onset of stimulus and the proper voluntary response. It involves stimulus processing, decision making and response programming. It is an indirect index of processing capabilities of the central nervous system.
The present study was carried out to bridge the relationship between dietary habits and endogenous sex hormone mediated sensory motor association and heart rate variability among young females during different phase of menstrual cycle.
Experimental Protocol
The present study was carried out on healthy volunteered 100 female medical students in the age group of 19-25 years with regular menstrual cycle. Ethical clearance was taken to perform this study in the department of Physiology of People’s College of Medical Science and Research Centre, Bhopal (PCMS/OD/2015/1069; IEC-2015/3). Subjects with irregular cycles, gynecological disorders, anaemia, history of drug intake affecting ANS and menstrual cycle or history of chronic diseases were excluded from the study. Different phase of menstrual cycle was sustained by questionnaire given to the subject. The selected students were divided into following groups based on their dietary habits. Subjects were classified into three diet groups, Group I (n=45): vegetarians, Group II (n=25); eggetarians and Group III (n= 30): non-vegetarians, where n denotes the number of individuals in each group. The mean age and BMI, of students was mentioned in our previous studies [8]. The present study examined audio-visual reaction time and HRV during all the three phases of menstrual cycle. They had a history of regular, predictable menstrual cycles within the preceding 6 months. All participants were free of medications during the study. After giving informed and written consent and receiving an orientation about the procedures of this study, participants began the study in the menses (Days 1-2 after menstrual onset), the follicular phase (7-10 days after menstrual onset), or the luteal phase (3-7 days before onset of the subsequent menstrual cycle). Participants were instructed to maintain their normal dietary pattern during each phase of their menstrual cycle. At early morning participants instructed for fasting state till experiments were carried out. Subjects were withdrawn from caffeinated beverages for at least 12 hours prior to the experiments and also would have been completed their night meal by 9 PM. They were also instructed to avoid active physical activity since the last evening before experiment.
Reaction Time Task
To examine the auditory and visual reaction time, a standard apparatus was used. It consisted of two components, a device for giving stimuli and another for responding stimuli, which were connected with an electronic device using audacity software for measuring the reaction time in milliseconds. During each examination procedure, the subjects were seated in front of a reception desk containing responding device. The task was to place the forefinger of the dominant hand on the central button, and as soon as the stimulus appeared to him, press the appropriate button. Each subject was told for respond to stimuli for both sound/light subsequently. Each experimental had done for three trials, from which the arithmetic mean was calculated and represented as an individual reaction time (milliseconds).
Heart Rate Variability Task
The recordings of ECG of all subjects were done by the single expert technician of our team in order to avoid any inter–observer error. A high quality ECG recording was taken under standardized condition to minimize artifacts, during the above mentioned three phases. To quantify heart rate variability, the analog ECG signal was measured for a period of 5 minute to obtain a QRS complex by using lead II. To minimize the aberration, the QRS complexes were filtered by high pass filter (40) and low pass filter (10). The obtained QRS complexes were amplified further for sufficient amplitude. The ECG signal was first analogally recorded and then digitally converted through an A/D converter to PC and analysed by using Digital data Acquisition system, HRV soft 1.1 VERSION. The measurements of HRV followed the standards suggested by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology set in 1996 [9]. The spectral components were the LF (0.04- 0.14 Hz) and HF (0.15-0.4 Hz), LF in normalized unit (LF nU), HF in normalized unit (HF nU), total spectral power in normalized unit and LF to HF ratio, was calculated. Time domain parameters like NN, SDNN, and RMSSD were also observed. Power spectral analysis of R-R interval variability has revealed that HF is modulated solely by the parasympathetic nervous system, whereas the LF component is together modulated by the sympathetic and parasympathetic nervous systems. Furthermore, the LF/HF ratio is also a useful parameter that reflects cardiac sympathetic modulations or sympatho-vagal balance as suggested by Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [9].
Statistical Analysis
Data are expressed as Mean±standard deviation (SD). All data were analysed with the SPSS for windows statistical package (version 20.0, SPSS Institute Inc., Cary, North Carolina. Statistical significance between the different groups was determined by one way-analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests when the groups showed significant difference and the significance level was fixed at p≤ 0.05. Finally, Pearson correlation coefficient was used to find correlation between two variables.
Results
Effect of Different Dietary Habits on Reaction Time
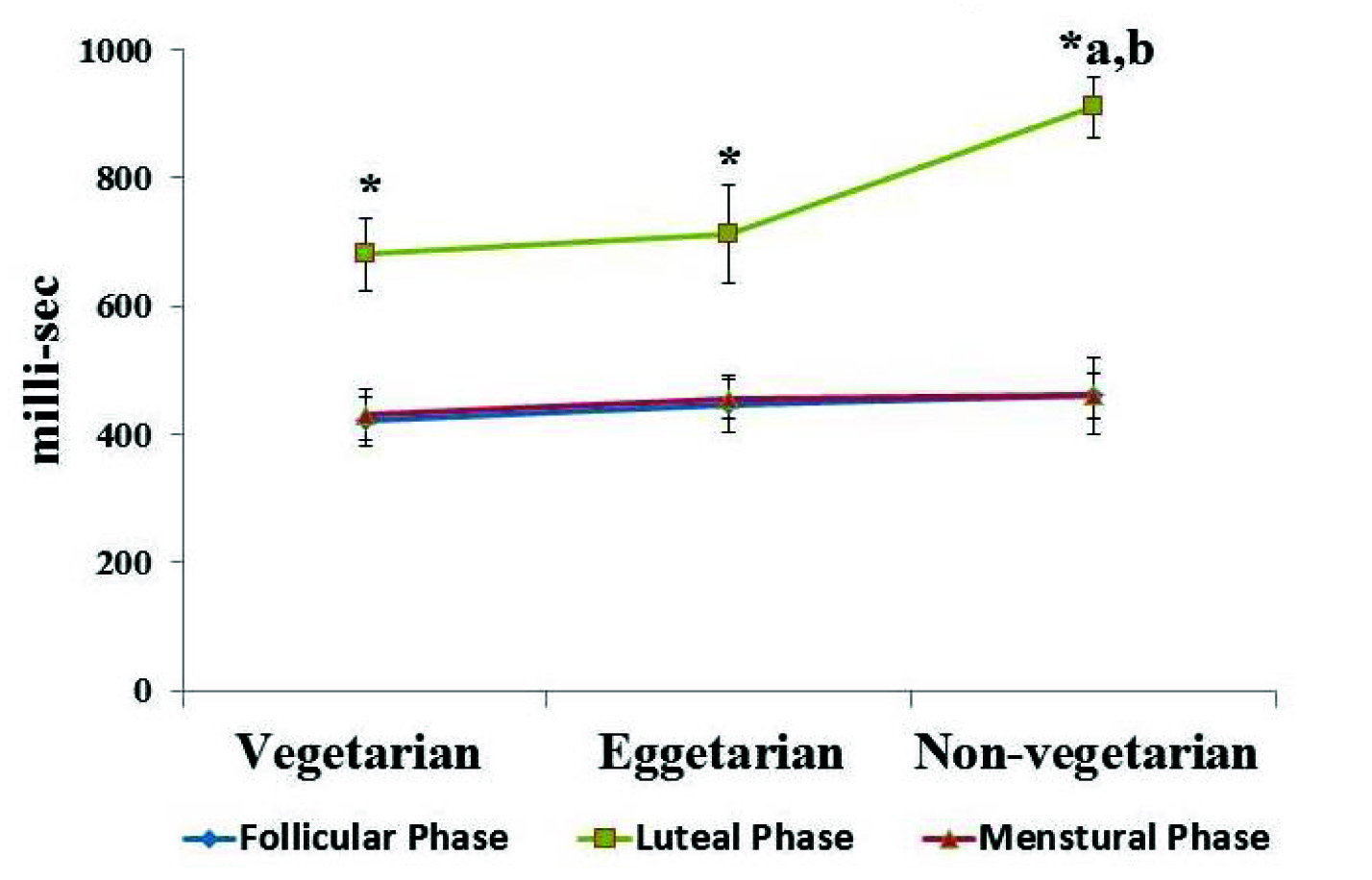
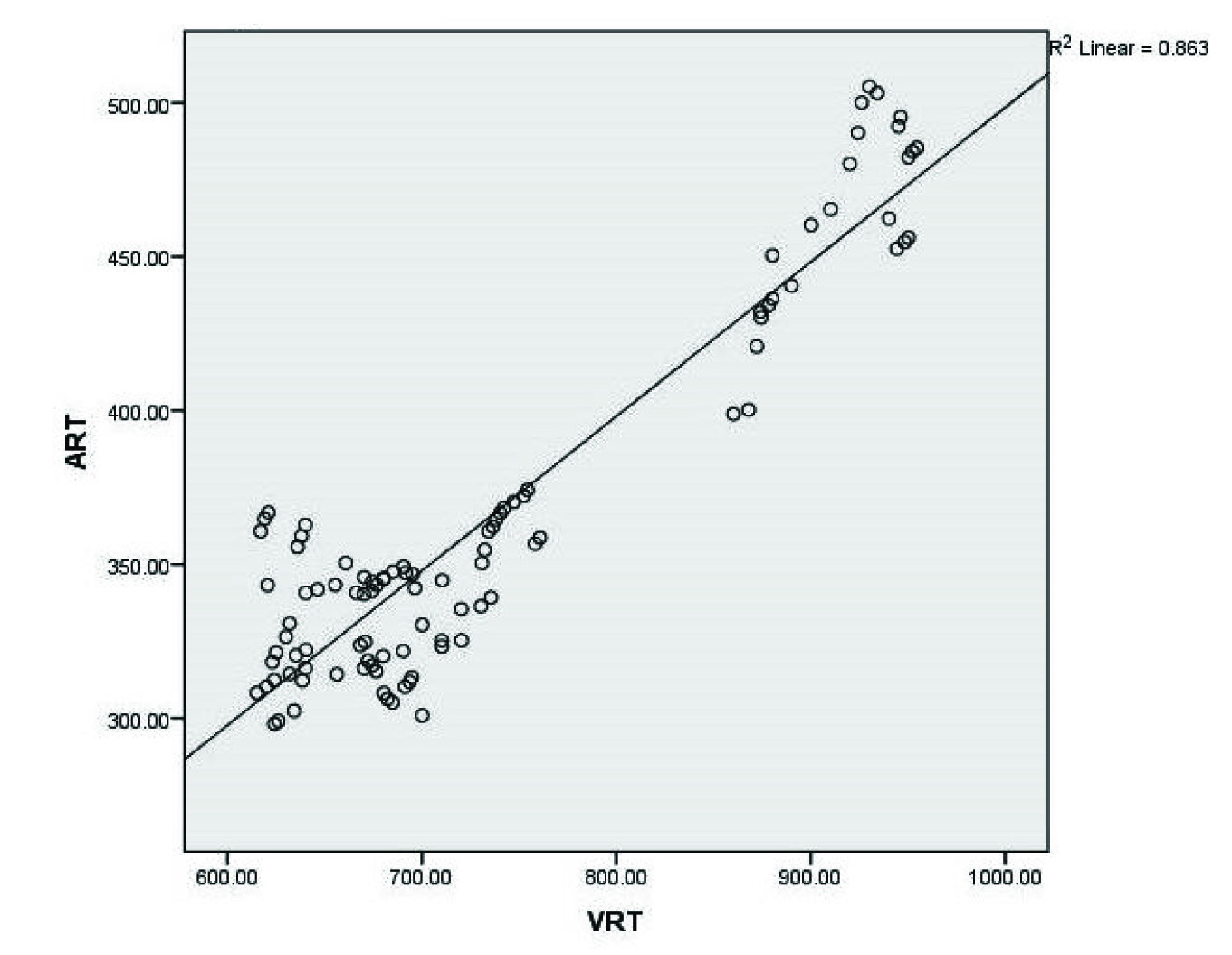
The data are summarized in [Table/Fig-1,2 and 3] with mean±SD. Among all the dietary habits (vegetarians, eggetarians and non-vegetarians) the visual and auditory reaction time was comparable in follicular and menstrual phase; however it was significantly prolonged in luteal phase when compared to follicular and menstrual phase. Moreover, among all the dietary habits, non-vegetarians showed more significant prolongation of visual and auditory reaction time in luteal phase when compared to vegetarians and eggetarians, as well as there was positive significant correlation {r(98)=+0.92; p= 0.01} between visual and auditory reaction time in luteal phase, among all the dietary habits [Table/Fig-4].
Effect of different dietary habit on visual reaction time, significance at p≤ 0.05, where, *Significant change compare with follicular phase and luteal phase, a – compared with vegetarians, b-compared with eggetarians.
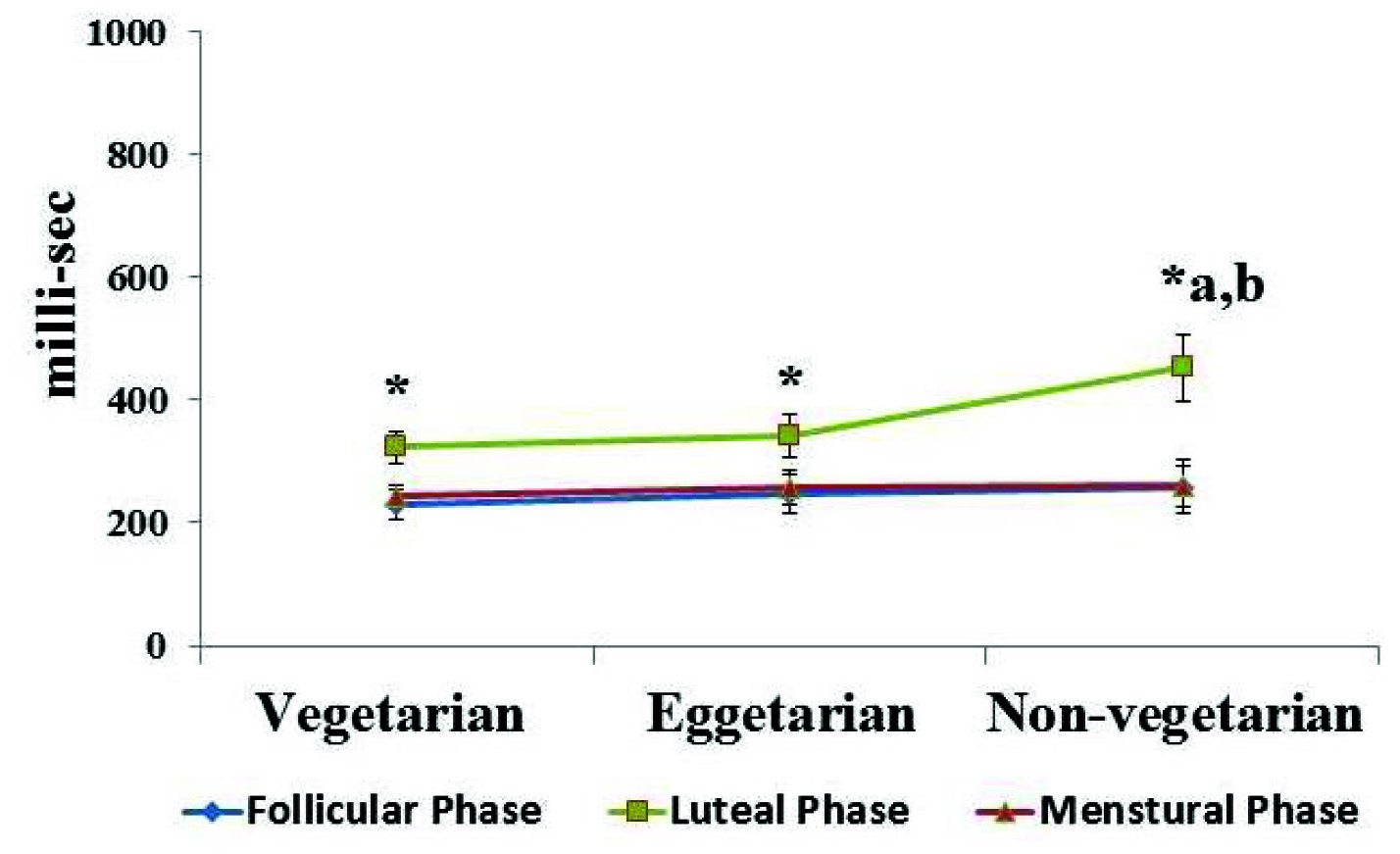
Effect of different dietary habit on auditory reaction time, significance at p≤ 0.05, where, *Significant change compare with follicular phase and luteal phase, a – compared with vegetarians, b-compared with eggetarians.
Correlation between visual reaction time (VRT) and auditory reaction time (ART) in luteal phase, among all dietary habits
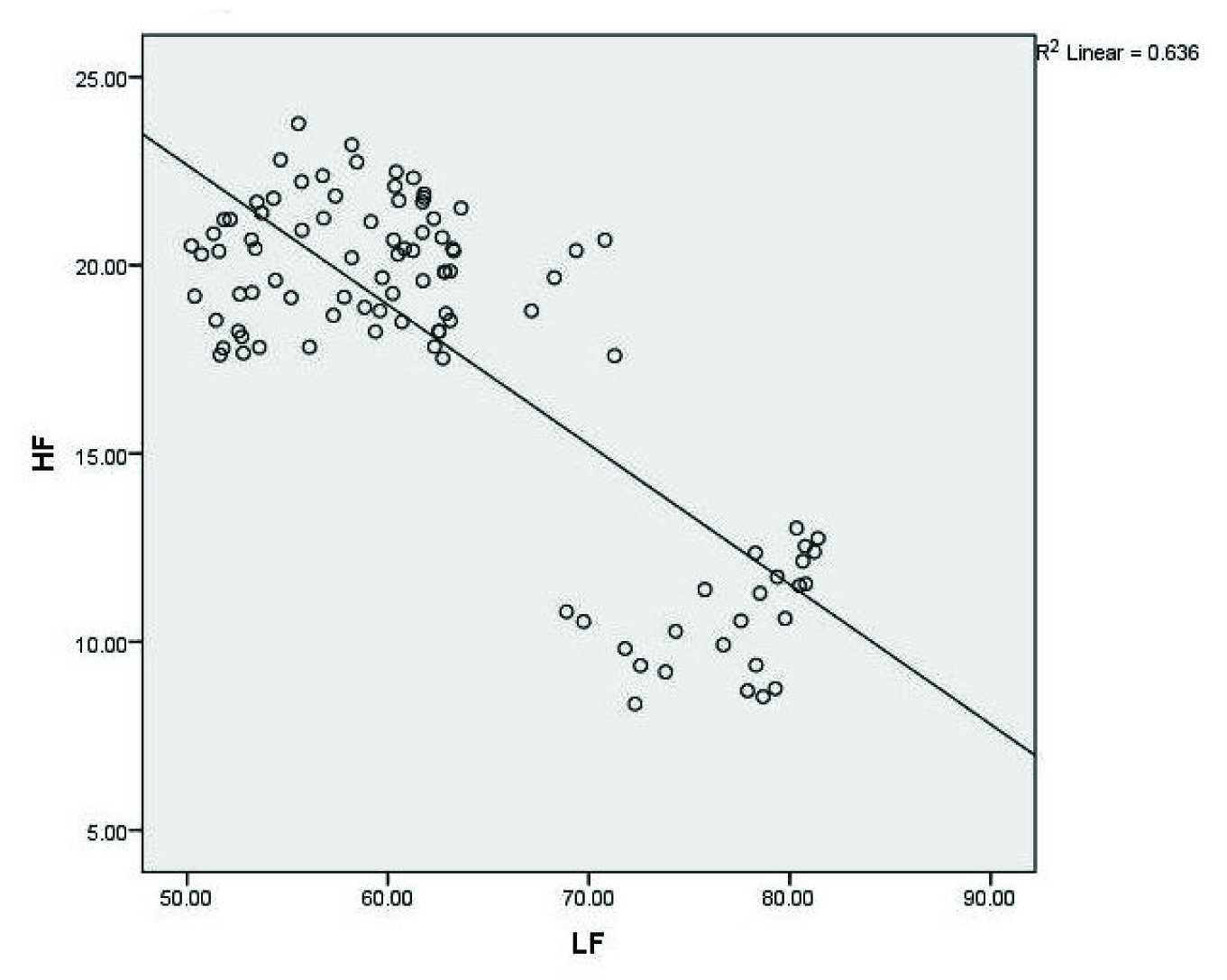
Correlation between LF and HF in luteal phase, among all dietary habits
Effect of different dietary habits on frequency domain parameters
The data are summarized in [Table/Fig-2,5] with mean±SD. There was not any significant alteration in the total spectral power (TSP), VLF, LF, HF and LF/HF ratio among all the dietary habit groups (vegetarians, eggetarians and non-vegetarians) in their follicular and menstrual phases, however, it was significantly decreased in TSP, HF and increased in VLF, LF, and LF/HF ratio in luteal phase when compared to follicular and menstrual phases. Moreover, among all the dietary habit groups, non-vegetarians showed more significant alteration of these parameters in luteal phase when compared to vegetarian and eggetarians, as well as there was negative correlation {r(98)=-0.79; p= 0.01} between LF and HF in luteal phase, among all the dietary habits.
Effect of dietary habits on time domain
| Parameter | Vegetarians | Eggetarians | Non-vegetarians |
|---|
| Mean NN(ms) |
| Follicular phase | 975±180 | 1074±195 | 1110±150 |
| Luteal phase | 1474±140* | 1650±184* | 2196±170*a,b |
| Menstrual phase | 985±163 | 1049±192 | 1110±212 |
| SDNN(ms) |
| Follicular phase | 45.15±5.24 | 47.60±4.38 | 43.24±3.15 |
| Luteal phase | 30.62±3.45* | 22.20±2.70* | 13.15±2.20*a,b |
| Menstrual phase | 44.27±4.30 | 46.27±4.05 | 42.33±5.44 |
| RMSSD(ms) |
| Follicular phase | 35.25±5.10 | 37.80±4.30 | 39.12±5.45 |
| Luteal phase | 25.18±3.80* | 23.10±2.15* | 15.24±3.14*a,b |
| Menstrual phase | 36.15±4.20 | 34.00±3.20 | 33.10±3.70 |
Significance at p≤ 0.05, where, *Significant change compare with follicular phase and luteal phase, a – compared with vegetarians, b-compared with eggetarians, (nu: normalized unit).
Effect of Different Dietary Habits on Time Domain Parameters
The data are summarized in [Table/Fig-6] with mean ±SD. The time domain parameters, NN interval, SDNN and RMSD did not show any significant alterations among all the dietary habit groups (vegetarians, eggetarians and non-vegetarians) in their follicular and menstrual phases, however, it was significantly increased in NN interval and decreased in SDNN, and RMSD in luteal phase when compared to follicular and menstrual phases. Moreover, among all the dietary habit groups, non-vegetarians showed more significant alteration of these parameters during luteal phase when compared to vegetarians and eggetarians groups.
Effect of dietary habits on frequency domain
| Vegetarians | Eggetarians | Non-vegetarians |
|---|
| Total spectral power(ms2) |
| Follicular phase | 3547±454 | 3380±314 | 3150±410 |
| Luteal phase | 2040±295* | 1776±255* | 1178±137*a,b |
| Menstrual phase | 3340±320 | 3113±247 | 2584±244 |
| VLF(ms2) |
| Follicular phase | 886±115 | 924±40 | 1030±190 |
| Luteal phase | 1274±137* | 1425±218* | 1980±225*a,b |
| Menstrual phase | 865±162 | 978±170 | 1010±213 |
| LF(ms2) |
| Follicular phase | 1092±185 | 1125±210 | 1177±272 |
| Luteal phase | 1710±258* | 1982±232* | 2710±290*a,b |
| Menstrual phase | 1157±170 | 1275±153 | 1325±235 |
| LF(nu) |
| Follicular phase | 40.24±7.12 | 43.14±6.20 | 45.00±9.10 |
| Luteal phase | 56.72±6.50* | 58.10±5.54* | 74.28±7.13*a,b |
| Menstrual phase | 42.12±5.80 | 45.22±4.40 | 47.19±7.80 |
| HF(ms2) |
| Follicular phase | 910±118 | 845±108 | 740±120 |
| Luteal phase | 596±80* | 528±95* | 320±57*a,b |
| Menstrual phase | 835±107 | 793±100 | 740±107 |
| HF(nu) |
| Follicular phase | 36.24±4.18 | 33.14±3.75 | 30.34±2.80 |
| Luteal phase | 20.52±3.24* | 19.54±2.14* | 10.47±2.55*a,b |
| Menstrual phase | 32.39±6.30 | 28.74±5.80 | 25.80±4.70 |
| LF/ HF |
| Follicular phase | 1.10±0.25 | 1.25±0.34 | 1.30±0.32 |
| Luteal phase | 2.20±0.30* | 2.32±0.25* | 3.45±0.41*a,b |
| Menstrual phase | 0.85±0.18 | 0.90±0.21 | 1.01±0.30 |
Significance at p≤ 0.05, where, *Significant change compare with follicular phase and luteal phase, a – compared with vegetarians, b-compared with eggetarians.
Discussion
In a previous study, we observed that non-vegeterian people are more prone to obesity (higher BMI) which may lead to early menarche and prolongation of menstrual cycle length [8]. In the present study we tried to evaluate nutritional habits that may influence sensorimotor association and heart rate variability (HRV) during different phases of menstrual cycle, by modulating the synthesis and metabolism of endogenous hormones (sex steroids) and their carrier proteins in the circulation. Reaction time evaluates the rapidity of central nervous system (CNS) for the coordination between the sensory and motor systems [10]. The interaction between sex hormones and neurotransmitter systems may result in cyclic changes in activation levels throughout the menstrual cycle. Autonomic nervous system is activated when sex hormone levels are low, while CNS activated during higher hormone levels [11]. Menstrual cycle is associated with dopamine release in the nigro-striatal pathway due to fluctuating levels of ovarian steroids hormones which may affect the brain regions [12]. The effects of estrogen on sensorimotor functions are attributed to its interaction with the dopaminergic system. During follicular phase progesterone production is low and estrogen levels increase gradually. At the end of follicular phase, there is exponential increase in levels of estrogen. After ovulation, progesterone levels also begin to rise. During luteal phase, both estrogen and progesterone are increased [13]. Prolongation of reaction time (visual and auditory) in follicular phase may due to exponential increase in levels of estrogen. Estrogen may enhance the inhibitory effects of GABA by stimulating its secretion and thereby delaying conduction. The excitation effects of estrogens are explained by their effects on glutamate, excitatory neurotransmitters excreted from the cortical neurons [14] and involvement in the neuromodulator of the serotonergic system [15]. Equally, progesterone may also decrease the sensitivity of neurons [16]. During follicular phase, when progesterone levels are low, low levels of GABA are in occipital cortex and during the luteal phase; when excretion of progesterone increases, GABA levels also increases, this indicate that progesterone metabolites may affect the activity of GABA. [17]. Performance of perceptual, as well as finger dexterity, was found improved during phases of higher levels of estradiol and both estradiol and progesterone (late follicular and mid-luteal phase) compared with the menstrual phase, when levels of sex hormones are low [18]. It has been shown by previous studies, that female sex hormones act at the receptor level on the hippocampus and hypothalamus that alters the excitability of the neurons across different phases of the menstrual cycle [19,20].
Heart rate variability (HRV) was used to examine the sympatho-vagal balance. The low-frequency (LF) component was found to be higher and the high-frequency (HF) component to be lower during luteal phase than during the follicular and menstrual phases. The LF/HF ratio was also found to be significantly greater in the luteal phase than during the follicular and menstrual phases. This data supports previous findings regarding menstrual cycle [21]. In the present study, we observed non-vegetarians showed more significant changes as compared to eggetarians and vegetarians. These data suggest that sympathetic nervous activities are predominant in the luteal phase as compared to follicular phase, and this sympathetic dominance is more evident among non-vegetarians which may be due to their higher BMI. Previous studies on correlation between HRV and body mass index (BMI) had established that higher BMI scores were correlated to higher sympathetic and lower parasympathetic activities [22,23]. Our results support the previous finding; higher BMI is associated with lower parasympathetic activity as reflected by a higher NN interval and lower SDNN and RMSSD. The present data support findings of alterations of sympatho-vagal balance, related to higher BMI among non-vegetarian individuals. The vegetarians exhibited a significantly higher-frequency power of heart rate variability than non-vegetarian individuals. The vegetarians exhibited increases of cardiac vagal activity by significant higher total power, low-frequency (LF); and high-frequency (HF) of HRV compared with the non-vegetarian individuals [24]. Long-term vegetarian diets may help vagal regulation of the heart without increasing the sympathetic variations of the cardiovascular system [25]. A difference of a balance of ovarian hormones may be responsible for these changes of autonomic functions during the menstrual cycle [26].
Limitation
Limitations of our study are that we have not measured serum levels of estrogen and progesterone hormones during different phases of menstrual cycle. Although, the measurement of hormones were not done in this study, but, from present knowledge it can be expected that hormonal changes during normal menstrual cycle, mainly fluctuating levels of estrogen and progesterone might affect the sensorimotor association and regulation of cardiac autonomic tone.
Conclusion
We concluded that sensorimotor association and regulation of autonomic tone are modified in luteal phase as compared to follicular phase and menstrual phase; however non-vegetarians had showed more significant alterations as compared to eggetarians and vegetarians. These suggest that sympathetic nervous activities are predominant in the luteal phase as compared to follicular phase, and this sympathetic dominance is more among non-vegetarians which may be due to their higher BMI. The fluctuating levels of ovarian hormones (estrogen and progesterone) across the normal menstrual cycle might be responsible for these changes. Long-term vegetarian diets may help vagal regulation of the heart without increasing the sympathetic variations of the cardiovascular system. This fact can be taken into attention in neurological and behavioral assessment of women. This is a preliminary study; more authentications of these results can be confirmed by measuring the hormonal levels during menstrual cycle.
[1]. Fujiwara T, Nakata R, Current problems of food intake in young women in Japan: their influence on female reproductive functionReprod Med Biol 2004 3:107-14. [Google Scholar]
[2]. Wilson JD, Foster DW, Kronenberg HM, Larsen PR, Williams Textbook of Endocrinology 1998 9th edPhiladelphia PAW.B. Saunders Company [Google Scholar]
[3]. Maki PM, Rich JB, Rosenbaum RS, Implicit memory varies across the menstrual cycle: estrogen effects in young womenNeuropsychologia 2002 40:518-29. [Google Scholar]
[4]. Rawal K, Saini I, Comparative Analysis of Measuring Heart Rate Variability during Different Phases of Menstrual Cycle in Young Healthy WomenIJIEE 2014 4:62-66. [Google Scholar]
[5]. Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Power spectral analysis of heartrate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dogCirc Res 1986 59:178-93. [Google Scholar]
[6]. Leicht AS, Hirning DA, Allen GD, Heart rate variability and endogenous sex hormones during the menstrual cycle in young womenExperimental Physiology 2003 88(3):441-46. [Google Scholar]
[7]. Bai X, Li J, Zhou L, Li X, Influence of the menstrual cycle on nonlinear properties of heart rate variability in young womenAm J Physiol Heart Circ Physiol 2009 297(2):765-74. [Google Scholar]
[8]. Choudhary AK, Jiwane R, Alam, T, Sadawarte SK, Dietary habits and menarche among young female medical studentsNJPPP 2015 5:1-4. [Google Scholar]
[9]. Task Force of the European Society of Cardiology and the North American Society of Pacing and ElectrophysiologyHeart rate variability: standards of measurement, physiological interpretation and clinical useCirculation 1996 93:1043-65. [Google Scholar]
[10]. Shenvi D, Balasubramanian P, A comparative study of visual and auditory reaction times in males and femalesInd J Physiol Pharmacol 1994 38:229-31. [Google Scholar]
[11]. Asso D, Braier JR, Changes with menstrual cycle in psychophysiological and self-report measures of activationBiol Psychol 1982 15:95-107. [Google Scholar]
[12]. McEwen B, Estrogen action throughout brainRecent Prog Hormon Res 2002 57:357-84. [Google Scholar]
[13]. Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy womenEuropean Journal of Endocrinology 2004 150:161-71. [Google Scholar]
[14]. Smith MJ, Keel BA, Greenberg BD, Adams BA, Schmidt PJ, Rubinow DA, Wassermann EM, Menstrual cycle effects on cortical excitabilityNeurology 1999 53:2069-72. [Google Scholar]
[15]. Sherwin BB, Estrogen and mood in womenEndocrinologist 2005 15:180-85. [Google Scholar]
[16]. Das S, Gandhi A, Mondal S, Effect of premenstrual stress on audiovisual reaction time and audiogramIndian J Physiol Pharmacol 1997 41:67-70. [Google Scholar]
[17]. Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Cortical γ-aminobuturic acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorderArch Gen Psychiatry 2002 59:851-58. [Google Scholar]
[18]. Šimic N, Tokic A, Pericic M, Performance of fine motor and spatial tasks during the menstrual cycleArh Hig Rada Toksikol 2010 61:407-14. [Google Scholar]
[19]. McEwen BS, Davis PG, Parsons B, Pfaff DW, The brain as target for steroid hormone actionAnn Rev Neurosci 1979 2:65-73. [Google Scholar]
[20]. Curtis DR, Game CJA, Johnston GAR, McCulloch RM, Central effects of β-(p chlorophenyl)- γ-aminobutyricBrain RES 1974 70:493-99. [Google Scholar]
[21]. Princi T, Parco S, Accardo A, Radillo O, De Seta F, Guaschino S, Parametric evaluation of heart rate variability during the menstrual cycle in young womenBiomed Sci Instrum 2005 41:340-45. [Google Scholar]
[22]. Molfino A, Fiorentini A, Tubani L, Martuscelli M, Rossi Fanelli F, Laviano A, Body mass index is related to autonomic nervous system activity as measured by heart rate variabilityEur J Clin Nutr 2009 63:1263-65. [Google Scholar]
[23]. Koenig J, Jarczok MN, Warth M, Ellis RJ, Bach C, Hillecke TK, Body mass index is related to autonomic nervous system activity as measured by heart rate variability – a replication using short term measurementsThe Journal of Nutrition, Health and Aging 2014 18(3):300-02. [Google Scholar]
[24]. Fu CH, Yang CC, Lin CL, Kuo TB, Alteration of cardiovascular autonomic functions by vegetarian diets in postmenopausal women is related to LDL cholesterol levelsChin J Physiol 2008 51(2):100-05. [Google Scholar]
[25]. Fu CH, Yang CC, Lin CL, Kuo TB, Effects of long-term vegetarian diets on cardiovascular autonomic functions in healthy postmenopausal womenAm J Cardiol 2006 97(3):380-83. [Google Scholar]
[26]. Yildirir A, Kabakci G, Akgul E, Tokgozoglu L, Oto A, Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variabilityAnn Noninvasive Electrocardiol 2002 7(1):60-63. [Google Scholar]