Dental casting alloys with high and low noble metal contents are widely used in dentistry in various prosthetic restorations like crowns and bridges, porcelain-metal restorations and partial-denture framework. Since the introduction of base metal alloys, Both Ni-Cr and Co-Cr formulations have become increasingly used as compared with conventional noble metal alloys [1].
One of the most important requirements of dental casting alloys is that they should be biocompatible i.e., they should have the ability to withstand the oral fluids and should not release any harmful products into the oral environment. Identification and quantification of the released elements is the most relevant measure of biocompatibility. Systemic and local toxicity, allergy, and mutagenecity all result from elements in the alloy being released during corrosion [1]. The release of elements from dental casting alloys is directly related to adverse biological effects [1,2].
Noble metal alloys are considered to be ideal for dental prosthesis, as they are corrosion resistant and biocompatible [2]. The base metal alloys have superior mechanical properties when compared to noble or high-noble alloys but, these base metal alloys have disadvantages such as high corrosion in acidic environment, difficult to finish and polish, allergic reaction and difficult to solder and cast [3].
The amount of corrosion is directly related to alloy composition, manipulation, pH of the environment, surface roughness and other factors [4–7].
It has been reported that protein containing solutions can accelerate the release of alloys from the base metal alloys [6]. Few evidence supports concerns of casting alloys which cause systemic toxicity, this study aims to determine the influence of solution in which an alloy is submerged and the exposure time on the amount of Ni, Cr & Mo ions released from the Ni-Cr alloys and Co from the Co-Cr alloys [Table/Fig-1].
Materials and Methods
Preparation of samples
Specimens of each casting alloy nickel–chromium alloys [Table/Fig-2] (Wiron 99) and cobalt chromium alloys [Table/Fig-3] (Wirobond C) were prepared in the form of circular discs of 5mm diameter and 2 mm thickness. The wax patterns were invested in a phosphate bonded investment material (Ballasum, Bego). The mixing procedures and burnout schedules (nickel–chromium, cobalt chromium alloys: 900°C) were followed according to the manufacturer’s instructions. Then, the wax patterns were cast using a centrifugal casting unit using both the alloys. A total of 126 specimens were made from the Ni-Cr alloy and 42 specimens were made from Co-Cr alloy for immersion into different solutions. After casting, the specimens were divested and air-abraded with 50μ Al2O3 to remove the investment material. The specimens were ultrasonically cleaned.
Composition of Wiron 99 (BEGO Ltd., Germany) in % by weight.
| Ni | 65 |
| Cr | 22.5 |
| Mo | 9.5 |
| Nb | 1 |
| Si | 1 |
| Fe | 0.5 |
| Ce | 0.5 |
| C | max. 0.02 |
Composition of Wironium (BEGO Ltd., Germany) in % by weight.
| Co | 63 |
| Cr | 29.53 |
| Mo | 5 |
| Si | 1 |
| Mn | 0.5 |
| Fe | 0.5 |
| N | 0.3 |
| C | 0.17 |
Immersion of Samples into Artificial Saliva and Bovine Serum Albumin
Immersion solutions were artificial saliva and 3% BSA. Both of the specimens were prepared in the laboratory. Artificial Saliva did not contain any protein as it would act as a control group for comparing the difference in elemental release with protein and without protein.
Seven specimens were immersed for each of elements in 10ml solution of artificial saliva and bovine serum albumin for a period for one day, four weeks and seven weeks. Elemental analysis for nickel, chromium and molybdenum from Ni-Cr alloy and Co from Co-Cr alloy was made using atomic absorption spectrophotometer at three different time intervals: On first day, end of the fourth week and end of seventh week.
Results
[Table/Fig-4] T-test. Comparison of release of elements in two solutions.
T-test. Comparison of release of elements in two solutions.
| Group Statistics |
|---|
| GROUP | TIME | GRP | N | Mean | Std. Deviation | t |
|---|
| Nickel | Day 1 | BSA | 7 | .07486 | .022770 | .159000 |
| Artificial saliva | 7 | .07229 | .036193 | p=0.876 ns |
| Week 4 | BSA | 7 | .18786 | .106071 | 1.808000 |
| Artificial saliva | 7 | .11100 | .037341 | p=0.096 ns |
| Week 7 | BSA | 7 | .21871 | .101224 | 1.166000 |
| Artificial saliva | 7 | .16257 | .077322 | p=0.266 ns |
| Chromium | Day 1 | BSA | 7 | .01657 | .007743 | 1.629000 |
| Artificial saliva | 7 | .01114 | .004220 | p=0.129 ns |
| Week 4 | BSA | 7 | .65143 | .211631 | 3.991000 |
| Artificial saliva | 7 | .21929 | .193087 | p=0.001 vhs |
| Week 7 | BSA | 7 | 1.33629 | .171835 | 12.836000 |
| Artificial saliva | 7 | .45043 | .060745 | p=0.001 vhs |
| Molybdenum | Day 1 | BSA | 7 | .07500 | .027893 | 1.555000 |
| Artificial saliva | 7 | .04457 | .043634 | p=0.146 ns |
| Week 4 | BSA | 7 | .14214 | .040577 | 2.465000 |
| Artificial saliva | 7 | .10029 | .019276 | p=0.03 sig |
| Week 7 | BSA | 7 | .22571 | .058320 | 3.155000 |
| Artificial saliva | 7 | .14829 | .028535 | p=0.008 hs |
| Cobalt | Day 1 | BSA | 7 | .75229 | .078570 | 16.646000 |
| Artificial saliva | 7 | .21014 | .035381 | p=0.001 vhs |
| Week 4 | BSA | 7 | 2.67000 | .571095 | 11.175000 |
| Artificial saliva | 7 | .23571 | .077528 | p=0.001 vhs |
| Week 7 | BSA | 7 | 2.63886 | .496566 | 11.685000 |
| Artificial saliva | 7 | .34757 | .150272 | p=0.001 vhs |
ns = not significant
vhs = very highly significant
sig = significant
There was no significant difference in release of nickle in both the solutions.
Chromium release was seen at all time intervals. There was no significant difference of release in both the solutions on day 1 where as there was statistically very highly significant increased amount of release in BSA solution at the end of week four and week seven. Molybdenum release was seen at all time intervals. There was no significant difference of release in both the solutions on day 1 where as there was statistically significant difference of release at the end of week four and week seven.
Cobalt release was seen at all time intervals. There was statistically very highly significant increased amount of release seen in BSA solution at all time intervals [Table/Fig-5].
Mean release of elements in BSA and artificial saliva.
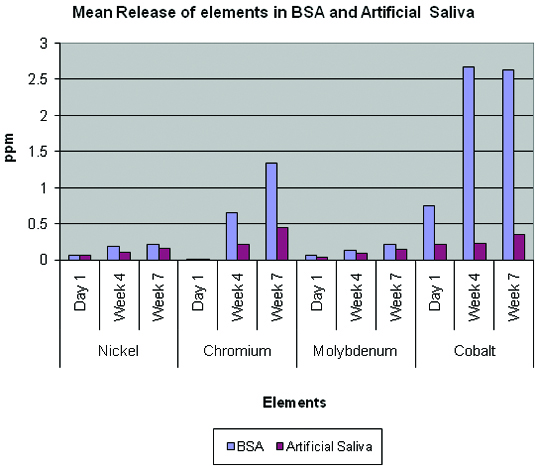
[Table/Fig-6] ANOVA Test. Comparision of the release of elements at different time intervals.
ANOVA test to compare the release at different time intervals.
| Descriptives |
|---|
| GROUP | GRP | | N | Mean | Std. Deviation | F | p |
|---|
| Nickel | BSA | Day 1 | 7 | .07486 | .022770 | | |
| Week 4 | 7 | .18786 | .106071 | | |
| Week 7 | 7 | .21871 | .101224 | 5.471 | 0.014 sig |
| Artificial saliva | Day 1 | 7 | .07229 | .036193 | | |
| Week 4 | 7 | .11100 | .037341 | | |
| Week 7 | 7 | .16257 | .077322 | 4.962 | 0.019 sig |
| Chromium | BSA | Day 1 | 7 | .01657 | .007743 | | |
| Week 4 | 7 | .65143 | .211631 | | |
| Week 7 | 7 | 1.33629 | .171835 | 122.999 | <0.001 vhs |
| Artificial saliva | Day 1 | 7 | .01114 | .004220 | | |
| Week 4 | 7 | .21929 | .193087 | | |
| Week 7 | 7 | .45043 | .060745 | 24.738 | <0.001 vhs |
| Molybdenum | BSA | Day 1 | 7 | .07500 | .027893 | | |
| Week 4 | 7 | .14214 | .040577 | | |
| Week 7 | 7 | .22571 | .058320 | 20.551 | <0.001 vhs |
| Artificial saliva | Day 1 | 7 | .04457 | .043634 | | |
| Week 4 | 7 | .10029 | .019276 | | |
| Week 7 | 7 | .14829 | .028535 | 18.311 | <0.001 vhs |
| Cobalt | BSA | Day 1 | 7 | .75229 | .078570 | | |
| Week 4 | 7 | 2.67000 | .571095 | | |
| Week 7 | 7 | 2.63886 | .496566 | 43.759 | <0.001 vhs |
| Artificial saliva | Day 1 | 7 | .21014 | .035381 | | |
| Week 4 | 7 | .23571 | .077528 | | |
| Week 7 | 7 | .34757 | .150272 | 3.759 | 0.043 sig |
There was a significant (p=0.014) more amount of Ni, Cr, Mo and Co release from day 1 to week 7 in BSA and significant(p=0.019) amount was also noted in artificial saliva.
[Table/Fig-7] ANOVA Test. Comparison of the release of elements from Ni-Cr alloy at different time intervals within a particular solution.
ANOVA test to compare the release of elements at different time intervals within a particular solution.
| Descriptives |
|---|
| TIME | N | Mean | Std. Deviation | F | p |
|---|
| BSA | Day 1 | Nickel | 7 | .07486 | .022770 | | |
| Chromium | 7 | .01657 | .007743 | | |
| Molybdenum | 7 | .07500 | .027893 | 17.575 | <0.001 vhs |
| Week 4 | Nickel | 7 | .18786 | 106071 | | |
| Chromium | 7 | .65143 | .211631 | | |
| Molybdenum | 7 | .14214 | .040577 | 28.903 | <0.001 vhs |
| Week 7 | Nickel | 7 | .21871 | .101224 | | |
| Chromium | 7 | 1.33629 | 171835 | | |
| Molybdenum | 7 | .22571 | .058320 | 201.237 | <0.001 vhs |
| Artificial saliva | Day 1 | Nickel | 7 | .07229 | .036193 | | |
| Chromium | 7 | .01114 | .004220 | | |
| Molybdenum | 7 | .04457 | .043634 | 6.091 | 0.01 hs |
| Week 4 | Nickel | 7 | .11100 | .037341 | | |
| Chromium | 7 | .21929 | .193087 | | |
| Molybdenum | 7 | .10029 | .019276 | 2.331 | .126 |
| Week 7 | Nickel | 7 | .16257 | .077322 | | |
| Chromium | 7 | .45043 | .060745 | | |
| Molybdenum | 7 | .14829 | .028535 | 58.214 | <0.001 vhs |
The release of elements Ni, Cr and Mo in BSA was statistically very highly significant (p<0.001) on day one, week four and week seven, with a increased release of Cr when compared to Ni and Mo [Table/Fig-8].
Comparison of Mean Release of elements from Ni-Cr alloy.
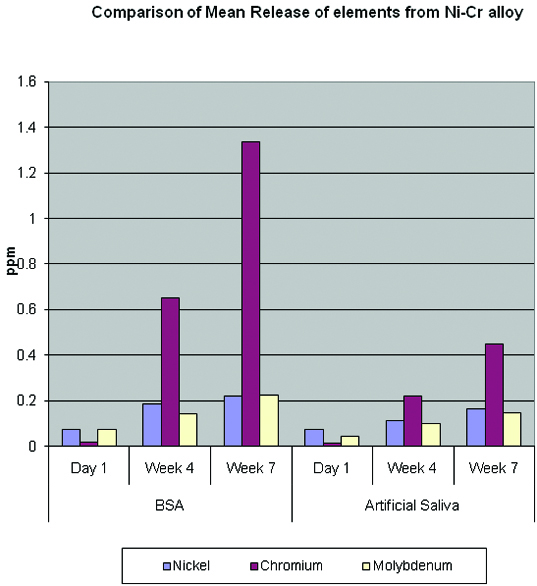
The release of elements Ni, Cr and Mo in Artificial Saliva was statistically significant on day 1, and week 7 where as at the end of week 4 the release was found to be not significant [Table/Fig-8].
[Table/Fig-9] TUKEY HSD Test. Multiple Comparison of release of elements in a particular solution.
TUKEY HSD for comparison between the elements in a particular solution. Dependent Variable: BSA Tukey HSD.
| Multiple Comparisons |
|---|
| TIME | TL1E | (I) GROUP | (J) GROUP | Mean Difference (-J) | p |
|---|
| BSA | Day 1 | Nickel | Chromium | .05829 | .083 |
| Molybdenum | -.00014 | 1.000 |
| Chromium | Molybdenum | -.05843 | .082 |
| Week 4 | Nickel | Chromium | -.46357 | 0.046 sig |
| Molybdenum | .04571 | .992 |
| Chromium | Molybdenum | .50929 | 0.025 sig |
| Week 7 | Nickel | Chromium | -1.11757 | <0.001 vhs |
| Molybdenum | -.00700 | 1.000 |
| Chromium | Molybdenum | 1.11057 | <0.001 vhs |
| Artificial saliva | Day 1 | Nickel | Chromium | .06114 | 0.011 sig |
| Molybdenum | .02771 | .426 |
| Chromium | Molybdenum | -.03343 | .268 |
| Week 4 | Nickel | Chromium | -.10829 | .251 |
| Molybdenum | .01071 | .998 |
| Chromium | Molybdenum | .11900 | .183 |
| Week 7 | Nickel | Chromium | -.28786 | <0.001 vhs |
| Molybdenum | .01429 | .991 |
| Chromium | Molybdenum | .30214 | <0.001 vhs |
On day one there was no significant difference between the release of elements in BSA. At the end of week 4 there was a significant difference found between nickel and chromium with a mean difference of -0.463ppm and chromium and molybdenum with a mean difference of 0.509ppm. At the end of week seven there was statistically very highly significant difference in release between Ni and Cr with a mean difference of -1.117ppm and chromium and molybdenum with a mean difference of 1.110ppm.
On day one there was a significant difference between the release of Ni and Cr with a mean difference of 0.061ppm in artificial saliva. At the end of week four there was no significant difference found between the elements. At the end of week 7 there was statistically very highly significant difference in release between Ni and Cr with a mean difference of -0.287ppm and chromium and molybdenum with a mean difference of 0.302ppm.
Discussion
The biocompatibility of dental casting alloys is a critical issue because these alloys are in long-term intimate contact with oral tissues and are subjected to a variety of chemical environment in the mouth. The dynamic natures of intraoral conditions cause corrosion [8,9].
An alloy is mixture of metals, usually two or more. Alloys used in dentistry usually contain four metals, and often 6 or more. Hence these alloys are complex in nature and because of this complexity and diversity, understanding their biocompatibility is difficult as any of the elements may be released which may influence the human body [2]. Elements that are released from alloys into the oral cavity may gain access into the body through the gut, through the gingiva or other oral tissues or, through the lungs. The route by which an element gains access inside the body is critical to its biologic effects. Once inside the body, metal ions can be distributed to many tissues, through the lymphatic system, or the bloodstream or may also be ingested by cells such as macrophages.
The absorption, distribution, retention half-life, and excretion are significantly influenced by the form of metal. The distribution of a metallic element is also critical to its ability to cause systemic toxicity. Ultimately, the body generally eliminates metals through the urine, feces, or lungs. It must be stressed that, if an alloy releases amounts of various elements equivalent to dietary intake, it does not implicate these alloys as having systemic biologic toxicity. In most situations, the amounts of elements that are released from dental alloys are below those taken in as a part of the diet. This result is not surprising when the normal daily dietary intake of metals in dental alloys is considered [2].
The daily intake (in μg) of some elements in dental alloys which are included in the diet are estimated to be chromium 240 μg, cobalt 250 μg, molybdenum 400 μg, nickel 400 μg.
Nickel is one of the most common allergens and the most potent sensitizer of all metals. The prevalence of nickel sensitivity in the general population has been estimated to range from 6.7% to 17.5%. It has been recommended that patients who are highly sensitive should not exceed a nickel concentration intake threshold of 0.06 mg/l. The amount of nickel in one dose that will induce an allergic response has been calculated as 0.6 to 2.5 mg. Although Ni is thought to be an essential micronutrient, intracellularly it binds to and depolymerise RNA and proteins, as well as disrupting muscle contractility and enzyme function [10].
Many studies have been carried out by different researchers about the release of metallic elements from dental alloys with many different materials and methods [4–6,11,12].
Two different immersion solutions for elements were used in this study: Artificial saliva and BSA. Artificial Saliva was laboratory prepared and its composition did not contain any protein in it as would act as a control group in this study to evaluate whether there was any difference in the release of elements with and without protein in the solutions. The artificial saliva used in this study is a less aggressive environment [5,13].
This study demonstrates that the biological environment affects significantly the corrosion of dental casting alloys. Immersion of the specimens showed there was release of the elements Ni, Cr, Mo and Co in both the solutions.
Generally, more elemental release from alloys occurred in BSA. This result is in accordance with previous study by John C Wataha et al., [6]. It has been previously reported that protein-containing solutions could accelerate the release of elements from alloys. Proteins can interact with the corrosion reactions in two ways: They can bind to metal ions and transport them away from the interface, therefore encouraging further dissolution; or proteins may be absorbed onto the metal surface, thus restricting the diffusion of oxygen to the surface and making it harder for the surface to repassivate [14].
Release of Ni was seen in both the solutions and there was no significant difference of release in two solutions. This result is in accordance with previous study by John C Wataha et al., [6]. The probable reason may be due to concentration of Cr. It is said that 16 to 27 percent can provide an optimum corrosion resistance for the nickel base alloys. In addition, Mo plays an active role in the formation of the oxide layer. Thus, an increasing concentration of Cr and Mo on the surface layer may synergistically lower the dissolution rates of metals [7,15]. This result is also in contrast to some studies which may be due to the less concentration of Cr in the alloy [5,7,15].
Nickel concentration of 112 ppm was said to be high enough to cause a positive reaction when Ni patch testing was performed [13], the patch test threshold for Cr was found to be ranging between 10ppm to 50ppm [16]. In this study the mean concentration of Ni release in the BSA solution was found to be 0.074ppm, 0.187ppm and 0.218ppm at day one, week four and week seven and for Cr it was 0.016ppm, 0.651ppm and 1.33ppm respectively which were any time less than the above mentioned level.
Cobalt is an essential element for life in small quantities. Median lethal dose (LD50) values of soluble cobalt salts have been found to be between 150 and 500 mg/kg. Next to nickel and chromium, cobalt is a major cause of contact dermatitis and is carcinogenic [17].
The release of elements Ni, Cr and Mo in BSA was statistically very highly significant (p<0.001) on day one, week four and week seven, with an increased release of Cr when compared to Ni and Mo. The release of elements Ni, Cr and Mo in artificial saliva was statistically significant on day one, and week seven where as at the end of week four the release was found to be not significant i.e., on the day one release was dominated by Ni whereas by the end of week four and week seven maximum concentration seen was of Cr. This difference in elemental release when compared to the other studies which predominantly were dominated by release of Ni can be due to an increasing concentration of Cr and Mo on the surface layer which may synergistically lower the dissolution rate of Ni [7,13].
Release of Co was seen in both the solutions at all time intervals and there was very high significant difference of release in two solutions on the first day, fourth week and at the end of seventh week with increased release in BSA. The Co release from the Co-Cr alloy was more at all time intervals when compared to the Ni from Ni-Cr alloy. This is in agreement with the study done by F. Nejatidanesh et al., who stated that the Ni-Cr alloys were more resistant to corrosion when compared to Co-Cr alloy [13].
The release of Ni, Cr, Mo and Co from BSA and artificial saliva was seen at all the time intervals. Wataha measured the release of elements from dental alloys for 10 months at a monthly interval [18]. They hypothesized that release of elements should decrease with the exposure of time to the medium and that the cytotoxic effects of the alloys should also decrease. They also stated that the initial release rates were more which was in accordance to this study.
Ana Milheiro studied the influence of shape and surface treatment of palladium based dental alloys and found that the polished specimens released much lower amounts compared to rough surface specimens [19].
Limitation
Further studies need to be carried out with the highly polished surface of the castings and also comparison can be done between the release of elements invitro and invivo.
Within the limitations of this study the elemental release were well below the dietary intake level and never reached the threshold for allergic response.
Conclusion
Within the limitations of this in vitro study, the following conclusions could be drawn, the release of elements Ni, Cr, Mo and Co was observed in both solutions, with predominant release of Cr from Ni-Cr alloy. The composition of solutions played an important role in the release of elements from the alloys. The protein containing solution (BSA) showed maximum release of elements from Ni-Cr and Co-Cr alloys. The elements released never reached their threshold for toxic effects. Hence these alloys can be safely used in fabrication of prosthesis without any ill effects.
ns = not significant
vhs = very highly significant
sig = significant