Squamous Cell Carcinoma (SCC) reports for approximately 95% of all oral malignant neoplasms and for about 38% of entire malignant head and neck tumours and poses a major global health problem [1]. It is sixth most frequently diagnosed malignancy with higher incidence of mortality and morbidity rate noted worldwide [2]. The prognosis of SCC is linked to the proliferative activity of the tumour, the degree of differentiation and invasion and metastatic potential [3]. The last two processes constitute of multiple steps including degradation of the basement membrane and extracellular matrix, alterations in cell adhesiveness, tumour cell motility and angiogenesis [4,5]. Oral Squamous Cell Carcinoma (OSCC) is linked with high mortality because of its local, regional and distant spread which is due to its aggressive and highly invasive nature [6,7]. Around the world, the five year mortality rate of oral cancer is about 50%, which has not altered significantly in past 50 years despite of the advances in surgery, radiotherapy and chemotherapy. The reason behind this could be the diagnosis of the OSCC cases at late stages as no authentic diagnostic markers are available which detect the disease at early stage. In addition, OSCC has a very high recurrence rate, which imposes a challenge on identification of recurrence or second primary tumours [8,9].
Human Matrix Metalloproteinase (MMP) gene family comprises of more than 20 members who on the basis of their structure and substrate specificity are classified into collagenases, stromelysins and gelatinases. Normally, MMPs are required in remodelling the extracellular motion, in which the function is inherent to normal tissue growth and differentiation. They are zinc-dependent proteases capable of digesting several components of the extracelluar matrix. From the subgroup gelatinases, MMP-9 is associated with degradation of type IV collagen, the main component of basement membrane. It is suggested to be associated with basement membrane disruption along with an important role in the distant metastatic potential of carcinoma cells. In various malignancies, MMPs overexpression has been associated with tumour invasion. MMP-1, -2 and -9 have been discovered in a large proportion of SCCs arising in various degrees of epithelial dysplasia. Although a crucial risk factor for oral cancer is the presence of epithelial dysplasia, many dysplasias will not advance to malignancy [10–12].
Thus, in this study, Immunohistochemistry (IHC) was performed to detect the expression of MMP-9 in epithelium from normal oral mucosa, oral epithelial dysplasia (OED) and OSCC.
Materials and Methods
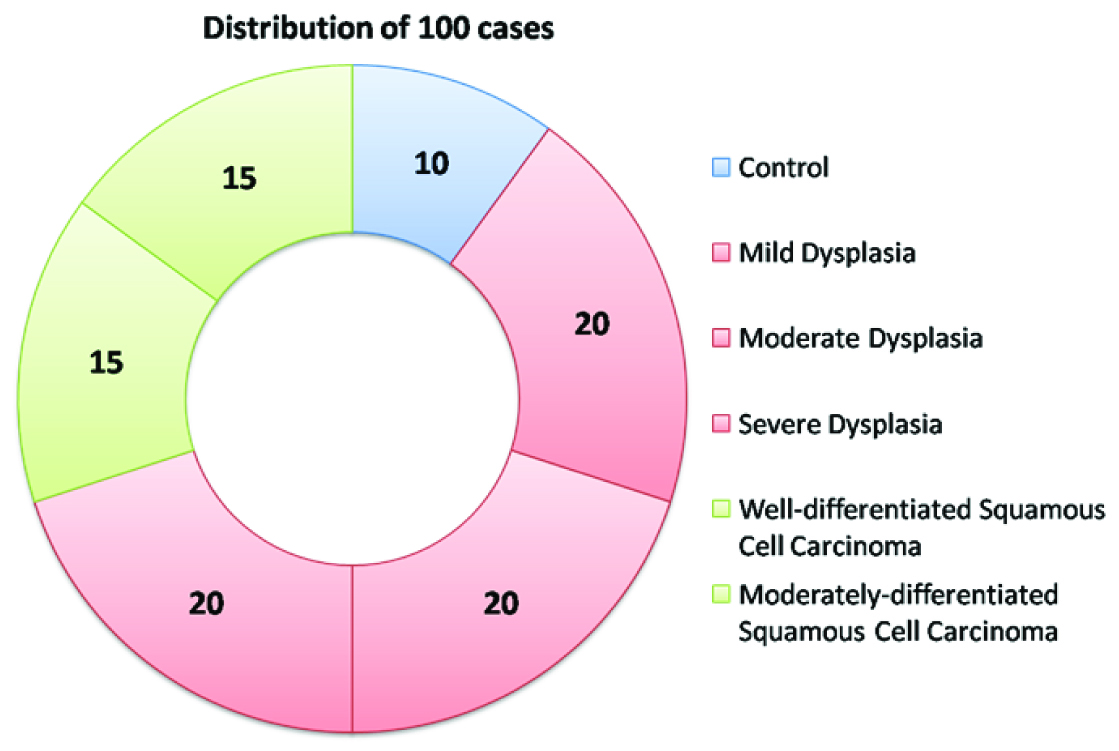
The study was carried out at the Department of Oral Pathology and Microbiology of M.M. College of Dental Sciences and Research, Mullana, Ambala from the year 2011 to year 2014 and the cases were collected from the archival samples. Formalin fixed paraffin embedded blocks of previously diagnosed cases of normal oral epithelium, oral epithelial dysplasia and oral squamous cell carcinoma were taken from the archives of the department. A total of 100 cases which were categorized into the three major groups: control, OED and OSCC as shown in [Table/Fig-1]. Inclusive criteria for the control group were those oral mucosal tissues with epithelium showing no dysplastic changes. From the paraffin embedded blocks of all the cases, two sections each of 4 μm thickness were obtained with Semi Automatic Microtome, with one set of albumin coated slides for Haematoxylin and Eosin staining and one set of poly-L-lysine coated slides for immunohistochemical staining. The slides were stained with MMP-9 antibody using immunohistochemical procedure. Haematoxylin and eosin staining was done in the other set for confirmation of diagnosis of the lesions and to confirm the presence of satisfactory tissue [Table/Fig-2].
Photomicrograph showing H&E staining in moderate dysplasia (X 400)

Immunohistochemistry
The sections of 4 μm thickness were obtained and mounted on poly-L-lysine coated slides. The sections were deparaffinised by keeping at 60°C on hot plate for 15 minutes and transferred to three changes of xylene and then hydrated through descending grades of ethyl alcohol. Antigen retrieval was done using citrate buffer (pH - 6.0) in EZ Retriever system in two cycles: Cycle 1: 980C for 5 minutes, Cycle 2: 950C for 8 minutes, followed by cooling to room temperature and then washed in phosphate buffered saline. The endogenous peroxidase activity was blocked using 0.3% peroxide stock solution for 10-15 minutes followed by a Phosphate Buffered Saline (PBS) wash. Power block was added for 10-15 minutes. Power block eliminates non-specific binding which can be caused by primary or secondary antibodies. The slides were then incubated with primary antibody MMP-9 (Biogenex, EP1255Y Rabbit monoclonal antibody in PBS with carrier protein and preservative). The slides were kept for overnight at 4±C in a moist chamber. The sections were then washed in PBS. After that the sections were incubated with secondary antibody (NovolinkTM HRP polymer). This step was followed by a PBS wash and then staining with DAB chromogen for 20 minutes till the brown colour appeared. The slides were put in distilled water and counterstained using Delafield’s haematoxylin. The slides were then dehydrated in graded ethyl alcohol, cleared in xylene for 5 minutes and mounted in DPX and viewed under Nikon microscope eclipse 80i.
Evaluation of Immunohistochemically Stained Tissue Sections
All stained sections were evaluated using 4X, 10X and 40X objectives of the Nikon microscope Eclipse 80i to demonstrate positivity for MMP-9 expression. Brown yellow particles in the cell membrane/cytoplasm were considered as positive staining [Table/Fig-3]. The expression was classified on the basis of the intensity of staining, the portion of the circumference of the cytoplasmic membrane stained and the percentage of cells exhibiting membranous staining. The sections were scanned at low magnification to identify positively stained areas (Hot Spots). Maximum of 10 hotspots in one slide (positive fields) were selected for qualitative score and the whole tissue section was considered for quantitative scores. In the control group and the Dysplasia group, analysis for positivity of epithelium was done in basal layer and spinous layer and the mean value of both layers were taken for analysis. In the carcinoma group, the analysis for positivity was done in central and peripheral islands of the tumour tissue. Similar to previous two groups, mean value of both central and peripheral islands of the tumour tissue were taken for analysis. Scoring was done according to the staining intensity (Qualitative score) and the ratio of positive cells (Quantitiative score). Qualitative score: 10 hot spot areas were evaluated at 40X and eventually at 100X to determine the colour shade of cytoplasmic particles and was scored as Score 0 - no yellow particles, 1 – light yellow, 2 – yellow brown and 3 - brown. Quantitative score: The whole section was evaluated at 100X to determine the percentage of positively staining cells which were evaluated as Score 0 – no staining, 1 - ratio < 25%, 2 – ratio 25-75% and 3 - ratio >75%. The final scoring was done as described by Shen LC, et al., [13]. The total score was taken as a multiplication product of both the Qualitative and quantitiative scores. Depending upon the total score, the final expression was graded as Negligible (0-2; +), Mild (3-5; ++), Moderate (6-8; +++) and Intense (9-12; ++++).
Photomicrograph showing MMP-9 expression in mild dysplasia arrow shows the presence of brownish yellow particles in cytoplasm (X 400)

Statistical Analysis
Data was scored and analysed. Mean, median and standard deviations for MMP-9 were calculated for all the groups. Data was further examined for statistical significance using SPSS 13.0 by Mann-Whitney Test and Kruskal-Wallis Test.
Results
Cytoplasmic staining was observed in all the three groups. [Table/Fig-4,5] show the mean value of MMP-9 score in all the three groups and subgroups. Minimum refers to the minimum value of the score and Maximum refers to the maximum value of the score. Using Mann-Whitney test for MMP-9 statistically significant difference was found between Group I and Group III (OSCC) (p <0.05), and highly statistically significant difference was found between Group II and Group III (p<0.001). Among the subgroups, statistically significant difference was seen between Group II b (Moderate dysplasia) and Group II c (Severe dysplasia) [Table/Fig-6,7].
Shows mean value of MMP-9 in all groups and subgroups
| Study groups | Subgroups | Minimum | Maximum | Mean ± SD |
|---|
| Group 1 (Control) | | 2 | 4 | 2.9 ± 0.875 |
| Group II(Dysplasia) | Mild Dysplasia IIa | 2 | 4 | 3.15 ± 0.812 |
| Moderate Dysplasia IIb | 2 | 4 | 2.85 ± 0.875 |
| Severe Dysplasia IIc | 2 | 4 | 3.4 ± 0.753 |
| Total | 2 | 4 | 3.13 ± 0.832 |
| Group III (OSCC) | Well Differentiated OSCC IIIa | 2 | 4 | 3.73 ± 0.593 |
| Moderately Differentiated OSCC IIIb | 3 | 4 | 3.86 ± 0.351 |
| Total | 2 | 4 | 3.80 ± 0.484 |
Graph showing mean value of all groups and subgroups

Shows significant p-value between Control vs OSCC and Dysplasia vs OSCC
| Mann-Whitney U | Asymp. Sig. (2 tailed) |
|---|
| Control vs. Dysplasia | 254.500 | 0.416 |
| Control vs. OSCC | 63.500 | 0.001* |
| Dysplasia vs. OSCC | 500.000 | <0.001** |
* Statistically significant; ** Highly statistically significant
Shows significant p-value between moderate vs severe dysplasia, severe dysplasia vs oscc and severe dysplasia vs moderately differentiated OSCC
| Mann-Whitney U | p-value |
|---|
| Control vs. Mild Dysplasia | 83.500 | 0.441 |
| Control vs. Moderate Dysplasia | 96.500 | 0.869 |
| Control vs. Severe Dysplasia | 67.500 | 0.123 |
| Mild vs. Moderate Dysplasia | 161.000 | 0.263 |
| Mild vs. Severe Dysplasia | 165.500 | 0.312 |
| Moderate vs. Severe Dysplasia | 130.500 | 0.045* |
| Well Differentiated OSCC v. Moderately Differentiated OSCC | 104.00 | 0.586 |
| Severe Dysplasia vs. OSCC | 212 | 0.027* |
| Severe Dysplasia vs. Well Differentiated OSCC | 112.500 | 0.136 |
| Severe Dysplasia vs. Moderately Differentiated OSCC | 99.500 | 0.039* |
* Statistically significant; ** Highly statistically significant
Discussion
In the epithelium of normal oral mucosa (control group), we observed mild to intense cytoplasmic expression of MMP-9 in basal and spinous layer. Out of 10 cases of normal oral mucosa, 40% case showed mild, while moderate and intense MMP-9 expression was shown by 30% of each of the subgroup [Table/Fig-8,9]. This was in accordance with Zhou et al., who observed MMP-9 expression in less than 10% of cells in specimens of normal epithelium [14]. Also, Fan et al., observed weak positive cytoplasmic MMP-9 expression in epithelium of normal tongue mucosa [15]. They observed MMP-9 expression in proliferating epithelial cells as brownish granules. On the contrary, Tsai et al., found faint immunoreactivity in normal epithelium [16]. Tamamura et al., could not find any positive MMP-9 expression in cases of normal epithelium [17].
Photomicrograph showing cytoplasmic MMP-9 expression in normal epithelium of oral mucosa (X 400)

Shows the number of cases showing Mild, Moderate and Intense staining in various groups and subgroups
| Groups and Subgroups | Total No. of Cases | No. of Cases Showing Negligible Staining | No. of Cases Showing Mild Staining | No. of Cases Showing Moderate Staining | No. of Cases Showing Intense Staining |
|---|
| Control | 10 | 0 | 4 | 3 | 3 |
| Mild Dysplasia | 20 | 0 | 5 | 7 | 8 |
| Moderate Dysplasia | 20 | 0 | 9 | 5 | 6 |
| Severe Dysplasia | 20 | 0 | 3 | 6 | 11 |
| Dysplasia (Total) | 60 | 0 | 17 | 18 | 25 |
| Well Differentiated OSCC | 15 | 0 | 1 | 2 | 12 |
| Moderately Differentiated OSCC | 15 | 0 | 0 | 2 | 13 |
| OSCC (Total) | 30 | 0 | 1 | 4 | 25 |
Normal oral mucosal keratinocytes unitedly with various epithelial cell lines expressed c-met protein which substantiated that normal oral epithelial cells have the machinery to react to Scatter factor. Scatter factor is a growth factor which is produced by a fibroblast. It acts in a paracrine manner on adjacent keratinocytes. It can induce MMP-9 expression in normal oral mucosal keratinocytes which can be reason for MMP-9 expression in normal oral mucosa [18].
In the group of oral epithelial dysplasia in our study, mild dysplasia cases showed 25% mild, 35% moderate & 45% intense MMP-9 expression. Moderate dysplasia cases showed 45% mild, 25% moderate & 30% intense MMP-9 expression while severe dysplasia cases showed 15% mild, 30% moderate & 55% intense MMP-9 expression. In total, oral epithelial dysplasia, 29% cases showed mild, 30% showed moderate & 41% showed intense MMP-9 expression [Table/Fig-3,9,10 and 11]. This was in accordance with Jordan et al., who observed overexpression of MMP-9 in 85% of cases [19]. Also, Fan et al., found increased MMP-9 immunoreactivity in cytoplasm of basal cells in dysplastic oral mucosal group [15].
Photomicrograph showing cytoplasmic MMP-9 expression in moderate dysplasia (X 400)

Photomicrograph showing cytoplasmic MMP-9 expression in severe dysplasia (X 400)

It is well accepted that oral epithelial dysplasia is a significant prognosticator of malignant development in potentially malignant lesions, and that particular architectural and cytological changes related with a heightened risk of malignant development have been identified as criteria essential to establish a diagnosis of oral epithelial dysplasia. According to Tamamura et al., MMP-9 gain of expression is related with collagen IV α chain loss and these are most probably vital events during transition from oral dysplasia to invasive cancer [17]. Also, it can be speculated that loss of type IV collagen α chains may have triggered the transition of oral precancer to invasive cancer through MMP-9 degradation of basal lamina. Many oral squamous cell carcinomas are preceded by red or white lesions showing varying degrees of epithelial dysplasia from mild to severe. Although a significant risk factor for oral cancer is the presence of epithelial dysplasia, many dysplasias will not advance to malignancy. Jordan et al., found that MMP-9 mRNA levels were significantly higher in oral dysplasias that advanced to cancer compared with those which did not, thus indicating that with increase in malignancy, a gain in MMP-9 expression is seen [19].
In OSCC group, we observed 6% mild, 14% moderate & 80% intense MMP-9 expression in well-differentiated OSCC while 14% cases showed moderate & 86% cases showed intense MMP-9 expression in moderately differentiated OSCC. In total, only 1% of case showed mild expression, 14% moderate & 85% intense MMP-9 expression for OSCC group [Table/Fig-9,12,13]. Similar results were observed by Kale et al., Zhou et al., Fan et al., Tamamura et al., Jordan et al., Ruokolainen et al., who found positive immunoreactivity to MMP-9 expression in majority of cases of squamous cell carcinoma [2,14,15,17,19,20].
Photomicrograph showing cytoplasmic MMP-9 expression in well-differentiated squamous cell carcinoma (X 400)

Photomicrograph showing cytoplasmic MMP-9 expression in moderately- differentitated squamous cell carcinoma (X 400)

Along with normal keratinocytes, in malignant keratinocytes as well, scatter factor stimulates MMP-9 expression and c-met which is a scatter factor receptor is expressed by these cells. MMP-9 and its principal substrates are basement membrane proteins laminin and collagen type IV. As the devastation of the basement membrane is a vital event in malignant transformation of oral epithelium, factors which determine synthesis of epithelial proteases are of potential meaning in tumour progression and scatter factor has a paracrine role in the ordinance of the protease activity [16]. MMPs are secreted at increased levels in cancer cells than by normal keratinocytes [18].
According to Fan et al., carcinomatous invasion is regulated by both initiators and promoters where intrinsic genetic changes in cancer cells are the ‘initiators’ of carcinogenesis and stromal cells are the ‘promoters’ [15]. Interaction or synergy between tumour cells and stromal cells in the enveloping microenvironment specifically between tumour cells and stromal fibroblasts and/or monocytes/macrophages can encourage tumour spread. They discovered that high MMP expression was found not only in tumour cells but also in stromal cells such as macrophages and vascular endothelial cells. As the tumour advances, stromal cells secrete MMPs that can damage basement membrane and extracellular matrix; they can also alleviate tumour spread via interaction with tumour cells.
MMPs are produced by cancer cells or through the induction of surrounding stromal cells [21]. According to Fullar et al., carcinoma associated fibroblasts are able to encourage the development of carcinoma cells [22]. Carcinoma-associated fibroblasts hasten an epithleial-mesenchymal transition in epithelial tumour cells, which is the main biological progression for squamous cell carcinoma. These cells produce pro-MMP-9 which is activated on the surface of tumour cells. When there is an interaction between carcinoma associated fibroblasts and tumour cells, it leads to the upregulation of MMP-9 in oral carcinoma cells.
The difference in MMP-9 expression between oral epithelial dysplasia and oral squamous cell carcinoma was found to be highly statistically significant. Between severe dysplasia and oral squamous cell carcinoma the difference in MMP-9 expression was found to be statistically significant. The difference in MMP-9 expression in well-differentiated squamous cell carcinoma and moderately-differentiated squamous cell carcinoma was found to be statistically insignificant [Table/Fig-7]. Thus, no correlation of MMP-9 expression was found with the histological grade. This was in accordance with Ruokolainen et al., who found MMP-9 expression to be independent of the stage or grade of the tumour [20]. Also, Zhou et al., observed no correlation between the status of MMPs and patient’s gender, age and the histopathological grade of their primary neoplasm [14].
Limitation
The main limitation of this study was that Poorly differentiated OSCC cases were not taken.
Conclusion
Thus, the present study suggested that MMP-9 expression is seen in normal, dysplastic as well as OSCC tissue. It increased from Normal Oral Mucosa Epithelium to Oral Epithelial Dysplasia and was seen maximum in Oral Squamous Cell Carcinoma in the cytoplasm of the epithelial cells. Thus, we suggest that MMPs are secreted at higher levels by cancer cells as compared to normal mucosa and dysplastic cells. Interaction between tumour cells and promoter cells increase the expression of MMP-9 which causes degradation of basement membrane and extracellular matrix; which facilitates tumour spread thus causing tumour progression.
* Statistically significant; ** Highly statistically significant
* Statistically significant; ** Highly statistically significant