Lady Windermere Syndrome: A Very Rare Entity In Indian Medical Scenario
Raghavendra Rao1, Shubha Sheshadri2, Navin Patil3, Karthik Rao4, Avinash Arivazhahan5
1 Assistant Professor, Department of Medicine, Kasturba Medical CollegeManipal University, Manipal, India.
2 Professor, Department of Medicine, Kasturba Medical College, Manipal University, Manipal, India.
3 Assistant Professor, Department of Pharmacology, Kasturba Medical College, Manipal University, Manipal, India.
4 Assistant Professor, Department of Medicine, Kasturba Medical College, Manipal University, Manipal, India.
5 Post Graduate, Department of Pharmacology, Kasturba Medical College, Manipal University, Manipal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Navin Patil, Assistant Professor, Department of Pharmacology, Kasturba Medical College Manipal University, Manipal-576104, India.
E-mail: navin903@gmail.com
Bronchiectasis is a common respiratory disorder, which we come across in clinical practice. Patients with bronchiectasis are prone to infections, especially of Mycobacterium tuberculosis. However, non-tubercular Mycobacterial infections may also set in, though rare. Here, we report an unusual case of Mycobacterium avium complex infection in a case of middle lobe bronchiectasis that was seen in a middle-aged immunocompetent female, a syndrome known as Lady Windermere Syndrome.
Bronchiectasis, Mycobacterium avium complex, Non-tubercular mycobacteria, Pulmonary, Tuberculosis
Case Report
A 48-year-old thin-built (BMI of 18.2 kg/sq.m.) female was admitted with history of recurrent episodes of cough with expectoration for the past 10 years, dyspnoea and fatiguability for the past 5 years, and pedal oedema for the past 10 days. There was no history of fever, postnasal drip, gastro-oesophageal reflux, anorexia, weight loss, chest pain, paroxysmal nocturnal dyspnoea, orthopnoea or palpitations. Also, there was no history of tuberculosis in the past. The patient was not a smoker.
On general examination, her vitals were stable. The patient had bilateral pitting pedal oedema, coarse crepitations in the right mammary area, loud S2 and ejection systolic murmur in the pulmonary area.
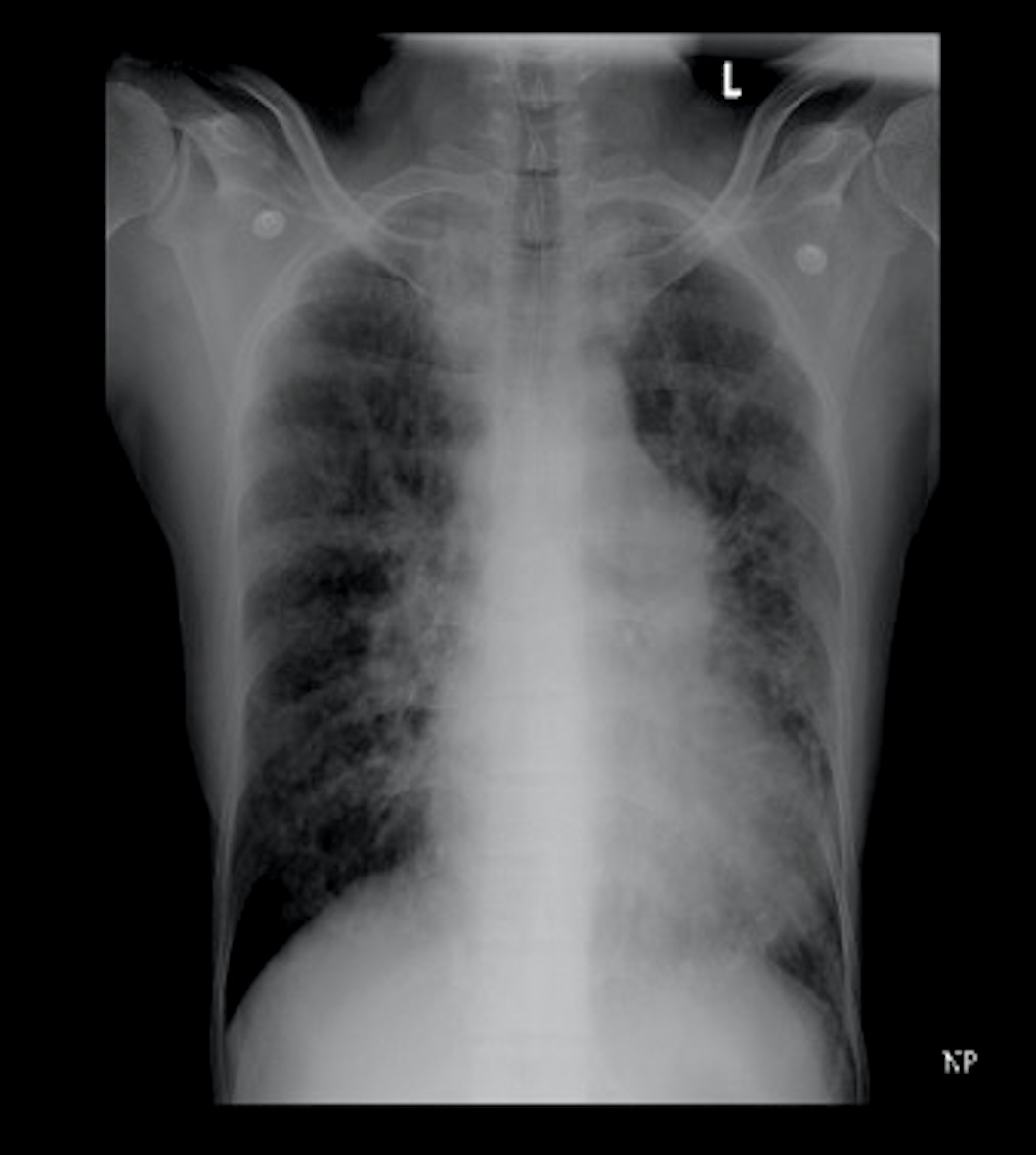
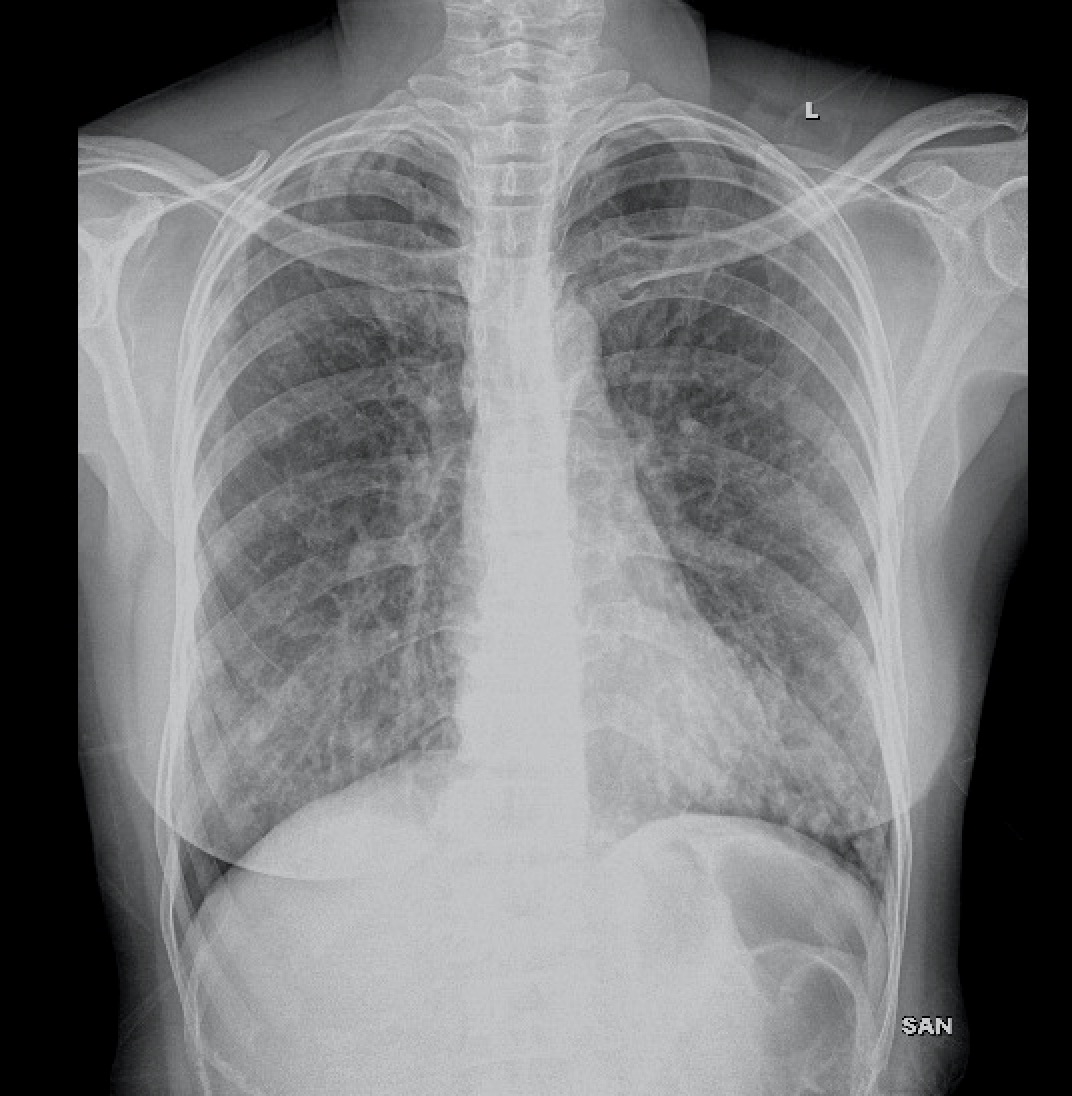
Basic laboratory evaluation showed complete blood profile, renal and liver functions to be normal. Chest X-ray showed evidence of right middle lobe bronchiectasis [Table/Fig-1]. Sputum culture showed no growth, and stain for acid-fast bacilli (AFB) was negative. The patient was also negative for HIV and hepatitis serological tests. Pulmonary function test showed evidence of small airway obstruction. Bronchoscopy findings were normal, except for secretions in the right middle lobe bronchus.
Chest X-ray showing right middle lobe bronchiectasis.
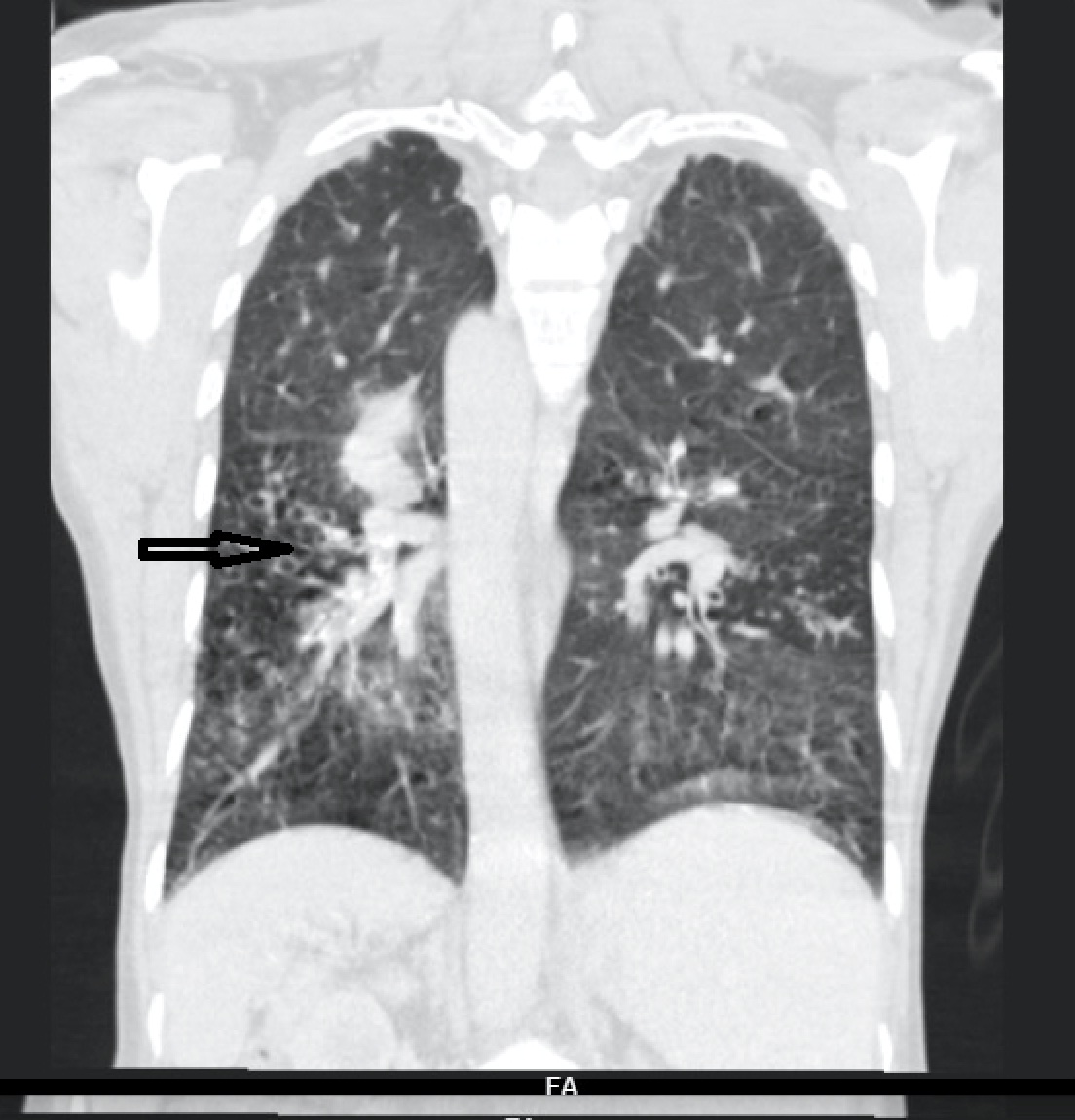
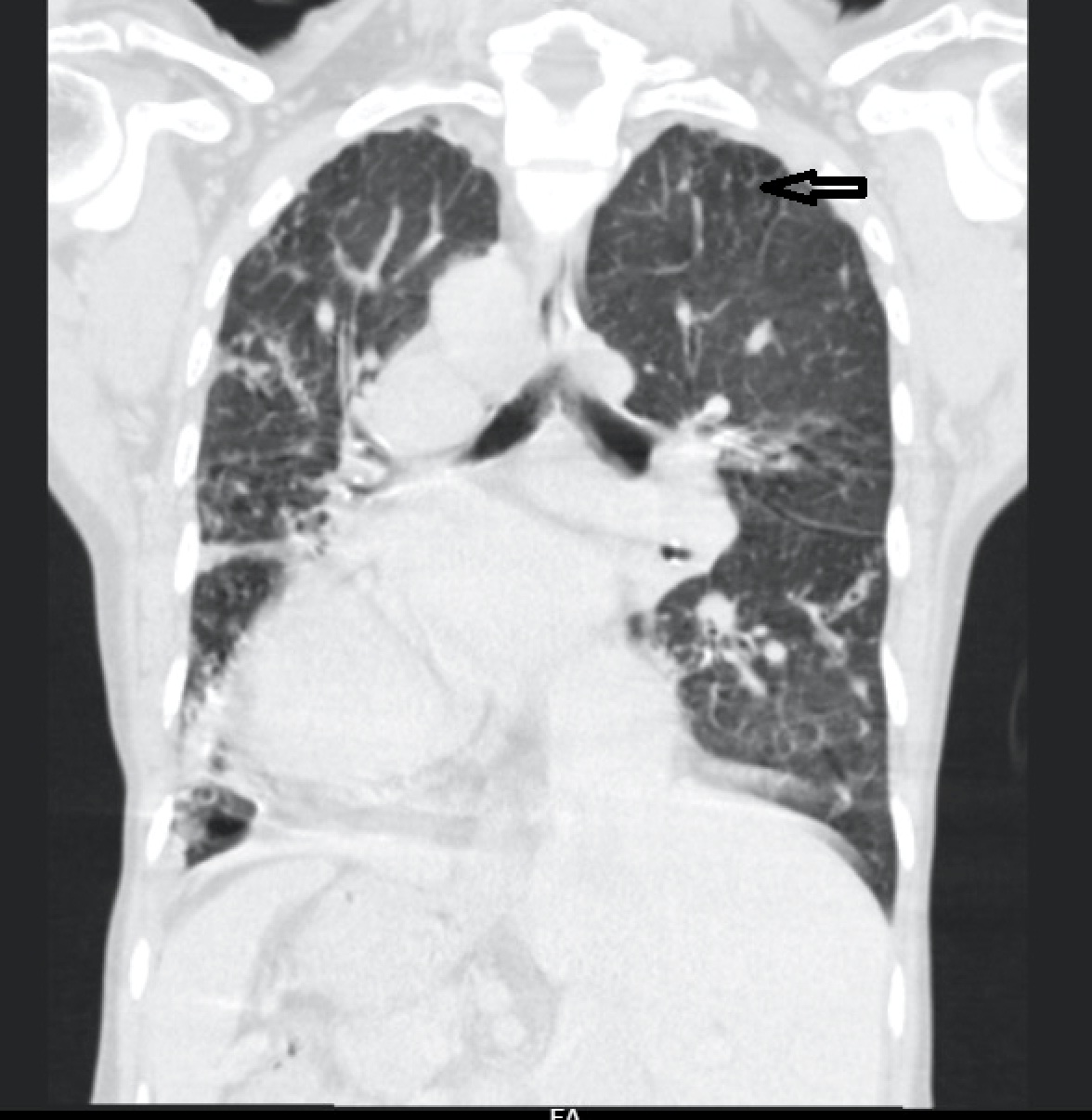
For further evaluation, a contrast-enhanced CT scan of the thorax was done, which revealed varicose bronchiectasis involving the right middle lobe [Table/Fig-2,3] and lingular bronchiectasis of the left upper lobe [Table/Fig-4]. Brochoscopic alveolar lavage fluid was sent for analysis, which grew strains of Mycobacterium avium complex (MAC). A 2D ECHO was done, which showed evidence of severe pulmonary artery hypertension and gross tricuspid regurgitation, with good right ventricular function. Also, the patient was asked if she had the habit of voluntarily suppressing cough, to which her response was affirmative. She was a habitual cough suppressor, as a mechanism to prevent episodes of upper abdominal pain on recurrent cough episodes. With all these findings observed, we concluded that it was a case of ‘Lady Windermere Syndrome’. We started the patient on a 3-drug regimen (rifampicin, ethambutol and clarithromycin). After 2 months of follow up, the patient’s symptoms had slightly improved [Table/Fig-5].
CECT thorax showing right middle lobe bronchiectasis.
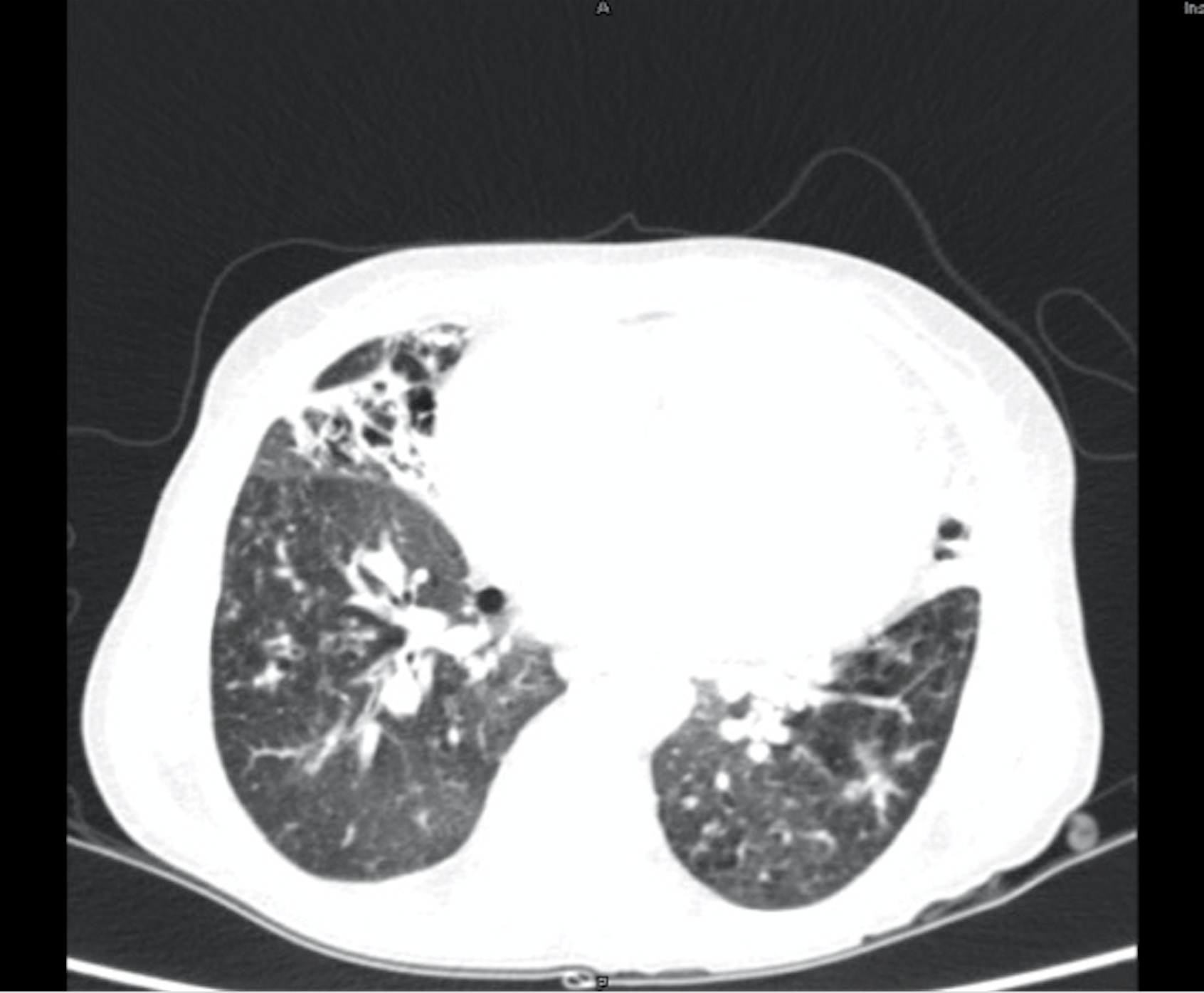
CECT thorax showing middle lobe bronchiectasis.
CECT thorax showing lingular bronchiectasis of left upper lobe.
Chest X-ray after 2 months of follow up.
Discussion
Runyon’s classification of NTM into four broad categories has been widely in use since its introduction. Mycobacterium avium complex (MAC) is an NTM that belongs to group 3 of this classification system [1]. MAC infections are most often seen in patients with pre-existing lung diseases. Bronchiectasis is one major risk factor for the acquisition of MAC infection. When a middle-aged woman presents with MAC infection following right middle lobe or lingular lobe bronchiectasis, the patient is diagnosed to have “Lady Windermere Syndrome” (LWS), the eponym commonly used for NTM infection [2].
Reich et al., first described Lady Windermere Syndrome (LWS) in 1992, based on observations from six patients, who were all elderly or middle-aged females [3]. Since then, there have been a few cases (mostly in white women) reported, chiefly from the West, and from Southeast Asia. To the best of our knowledge, we could not find reports of the syndrome in the Indian population in the available medical literature, making it a very rare entity in the population. Since no exact number of cases can be arrived at, it may not possible to know the incidence and prevalence in the population. Since India is home to several individuals affected with tuberculous bacteria, the number of patients with non-tuberculous lung disease will be lesser. Conversely, in the West, wherein the TB disease burden is negligible, the prevalence of NTM diseases is significantly higher. Another reason could be the distribution of the bacteria themselves, with the NTM being more commonly distributed in the West, as compared to India [4]. The syndrome is named after a fastidious character in one of Oscar Wilde’s plays, “Lady Windermere’s Fan”.
LWS appears to be more common in thin females, who have never smoked, and who have no underlying pulmonary disease [5]. Voluntary suppression of cough (hence, fastidious) in these women is hypothesized to cause reduced clearance of secretions from the right middle lobe and lingular segments, which have long and narrow bronchi with acute angulations, thus predisposing the patients to MAC infection [6–8]. This has been reported in several cases available in the literature. One such example is of a patient who used to suppress cough only to avoid the sternal pain that appeared after each episode of cough [9]. A similar scenario was experienced in our case as well.
Iseman et al., described most of their patients with LWS as having a high incidence of skeletal abnormalities (including pectus excavatum, mild scoliosis and straight back) [10]. This has been reported to be a common association in other reports as well [11]. Also, another commonly reported association is that with various connective tissue disorders, as evident from the literature [10]. However, these features were not seen in our patient, probably disproving the universal existence of such associations. Most of the reported cases were in thin and poorly built females, as was the case in the current scenario as well [12].
The diagnosis of LWS requires consistent symptoms with one of the following additional signs [1]:
Chest X-ray or CT scan showing evidence of right middle lobe (or left lingular lobe) lung infection.
Sputum culture or bronchoalveolar lavage culture demonstrating the infection caused by MAC.
LWS is usually treated with a three-drug regimen of clarithromycin (or azithromycin), rifampicin and ethambutol, with the total duration of therapy lasting for at least one year [1,5,13]. There have been reports of complete resolution at the end of pharmacotherapy for a year [14]. The drugs are commonly administered thrice daily, in view of better patient compliance [15]. However, a few patients may require surgical intervention in the form of thoracoscopic pulmonary resection [16].
Conclusion
Although in countries like India, pulmonary tuberculosis poses a major health burden, non-tubercular mycobacterial diseases should also be considered in atypical scenarios, wherein there is no TB contact history, or when the patient does not have classical symptoms of TB. Especially when the patient is a middle-aged or an elderly female patient with bronchiectasis, Lady Windermere syndrome has to be on the list of differential diagnoses.
[1]. Bonaiti G, Pesci A, Marruchella A, Lapadula G, Gori A, Aliberti S, Nontuberculous Mycobacteria in Noncystic Fibrosis BronchiectasisBiomed Res Int 2015 2015:197950 [Google Scholar]
[2]. Manglani RP, Khaja M, Hennessey K, Kennedy O, Pleural Mycobacterium Avium Complex Infection in an Immunocompetent Female with No Risk FactorsCase Rep Pulmonol 2015 2015:760614 [Google Scholar]
[3]. Reich JM, Johnson RE, Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern: The Lady Windermere syndromeChest 1992 101(6):1605-09. [Google Scholar]
[4]. Ioachimescu OC, Tomford JW. Nontuberculous Mycobacterial Disorders. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/nontuberculous-mycobacterial-disorders/ (Last accessed on November 21, 2015 at 17:00 hours) [Google Scholar]
[5]. Bhat SP, Nanda S, Kintzer JS, The Lady Windermere syndromePrim Care Resp J 2009 18(4):334-36. [Google Scholar]
[6]. Samjot SD, Chatrchai W, Lady Windermere Syndrome: Middle lobe bronchiectasis and Mycobacterium avium complex infection due to voluntary cough suppressionClin Infect Dis 2000 30(3):572-75. [Google Scholar]
[7]. Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Pulmonary Nontuberculous Mycobacterial Disease: Prospective Study of a Distinct Preexisting SyndromeAm J Respir Crit Care Med 2008 178(10):1066-74. [Google Scholar]
[8]. Reich JM, Cough suppression disorders spectrumRespir Med 2014 108(2):413-15. [Google Scholar]
[9]. Tryfon S, Angelis N, Klein L, Tsirikos-Karapanos N, Karipidis E, Antoniadis A, Lady Windermer syndrome after cardiac surgery procedure: a case of Mycobacterium avium complex pneumoniaAnn Thorac Surg 2010 89(4):1296-99. [Google Scholar]
[10]. Iseman MD, Buschman DI, Ackerson IM, Pectus excavatum and scoliosis: thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complexAm Rev Respir Dis 1991 144:914-16. [Google Scholar]
[11]. Simon P, Meurant F, Degives R, [Lady Windermere Syndrome]Rev Med Liege 2012 67(1):5-7. [Google Scholar]
[12]. Portillo K, Morera J, Nutritional status and eating disorders: neglected risk factors for nontuberculous mycobacterial lung disease?Med Hypotheses 2012 78(1):39-41. [Google Scholar]
[13]. Griffith DE, Aksamit T, Brown-Elliot BA, Catanzaro A, Daley C, Gordin F, An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseasesAm J Respir Crit Care Med 2007 175(4):367-416. [Google Scholar]
[14]. Chick JF, Chauhan NR, Bair RJ, Chauhan VR, The Lady Windermere SyndromeIntern Emerg Med 2013 8(1):83-85. [Google Scholar]
[15]. Jarand J, Field SK, Embrace simplicity when treating Lady WindermereChest 2014 146(2):244-46. [Google Scholar]
[16]. Yu JA, Pomerantz M, Bishop A, Weyant MJ, Mitchell JD, Lady Windermere revisited: treatment with thoracoscopic lobectomy/segmentectomy for right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial diseaseEur J Cardiothorac Surg 2011 40(3):671-75. [Google Scholar]