Spinal Intradural Schwannoma with Acute Intratumoural Haemorrhage: Case Report and Review
G. Lakshmi Prasad1, Lakshman I. Kongwad2, Manna G. Valiathan3
1 Assistant Professor, Department of Neurosurgery, Kasturba Medical College, Manipal University, Manipal, India.
2 Registrar, Department of Neurosurgery, Kasturba Medical College, Manipal University, Manipal, India.
3 Professor, Department of Pathology, Kasturba Medical College, Manipal University, Manipal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. G. Lakshmi Prasad, Department of Neurosurgery, Room 12, OPD Block, Kasturba Hospital, Manipal-576104, India.
E-mail: lakshmi.prasad@manipal.edu
Schwannomas account for around half of all intradural spinal tumours, with chronic progressive symptoms as the most common presenting features. Intratumoural haemorrhage as a presenting feature of spinal schwannoma is very rare and only 11 cases have been reported till date. Authors here report a previously asymptomatic 40-year-old male who presented with acute onset paraplegia 12 hours after a minor trauma. MR imaging revealed a C7-D3 intradural-extramedullary lesion with features of acute blood and showing no enhancement. Emergency laminectomy and complete removal of the mass was performed and histopathology revealed features of schwannoma with haemorrhage. Patient had modest improvement of his neurological deficits at a follow-up of 6 months. Pertinent literature is reviewed in brief.
Acute paraplegia, Nerve sheath tumour, Neurinoma, Thoracic
Case Report
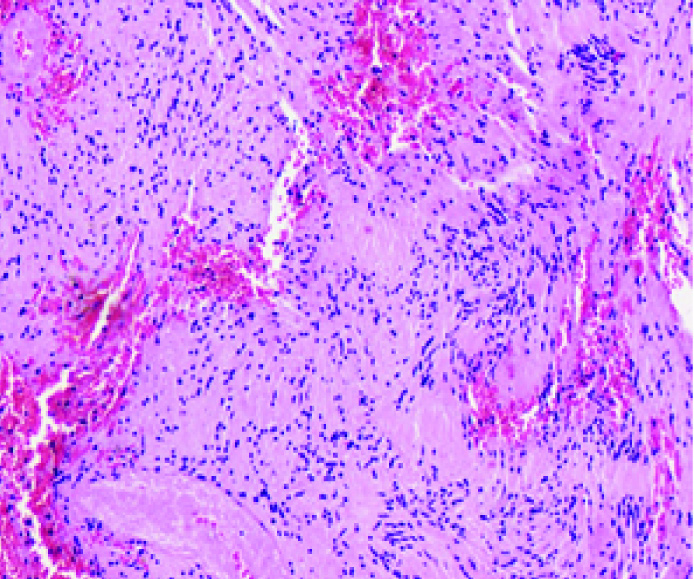
A 40-year-old male with no co-morbid illness presented with acute lower limb weakness progressing to paraplegia within 4 hours. He had suffered a minor fall 12 hours prior to his onset of weakness. There was no prior history of back ache or any other symptoms. At presentation to us, 2 days after the incident, neurological examination revealed mild flaccidity in bilateral lower limbs with grade 0/5 power, lower limb are flexia and sensory level at around D4 dermatome. There was no spinal column tenderness or deformity. MR imaging showed a C7-D3 lesion located in the intradural-extramedullary compartment compressing and displacing the cord anteriorly and to the left. The lesion was isointense on T1 and hypointense on T2 weighted sequences with no contrast enhancement, thus giving the appearance of acute blood [Table/Fig-1a-e]. There was evidence of neither a fracture nor cord signal changes on imaging. An emergency C7-D3 laminectomy was performed. Dural bulge was present and it possessed a reddish-hue which, after incising, a haemorrhagic extramedullary lesion was seen posteriorly compressing and displacing the cord to the left and anteriorly [Table/Fig-2a]. There was no evidence of subarachnoid haemorrhage and CSF was clear. There was a good arachnoid plane between the lesion and cord and it was attached to two dorsal nerve roots [Table/Fig-2b]. The roots were sacrificed and enbloc removal of the lesion was done which measured 6 cm in length [Table/Fig-2c]. The spinal cord was adequately decompressed after tumour removal [Table/Fig-2d]. Wound closure was done in standard fashion. At 6 months follow-up, he had modest improvement in limb weakness (grade 3 to 4-/5) and was ambulant with support. On H&E sections, the lesion showed intersecting facicles of spindled cells with hypo and hypercellular areas in a haemorrhagic background. In the hypercellular Antoni A areas, palisading of nuclei forming Verocay bodies was seen. There were no evidence of degenerative changes such as xanthomatous/ cystic changes, calcification, vascular hyalinization, nuclear pleomorphism and necrosis, which ruled out the possibility of an ancient schwannoma. Overall histopathological features were suggestive of a benign schwannona with intratumoural haemorrhage [Table/Fig-3].
Pre-op MRI. Axial and sagittal images showing a C7-D3 intradural-extramedullary lesion (arrows) which is isointense on T1 sequences (A,B) and hyperintense. on T2 sequences (C,D) on the right side compressing and displacing the cord to the left (arrowheads). Sagital contrast images showing no contrast enhancement of the lesion (E).

Intraoperative photographs: a) The dorsally situated large haemorrhagic intradural lesion can be seen displacing the cord anteriorly and to the left; b) 2 dorsal nerve roots are seen attached to the lesion (arrows in b); c) enbloc removal was done after sacrificing the roots and resected specimen measuring 6 cm in length; d) spinal cord has been adequately decompressed after tumour removal.

Histopathology-H&E x150 showing Verocay bodies in an extensively haemorrhagic background suggestive of a schwannoma
Discussion
Schwannomas account for around one-third of primary spinal neoplasms, occurring equally in both sexes [1–3]. They are most commonly located in cervical and lumbar regions because of the higher density of nerve roots at these sites [2,4]. The most common form of spinal haemorrhage is subarachnoid haemorrhage (SAH) and is usually caused by trauma, vascular malformations or coagulopathies [5,6]. Neoplasms with intratumoural haemorrhage are usually ependymomas, hemangioblastomas or cavernomas [5–7]. Intratumoural haemorrhage has been occasionally noted in cranial schwannomas; however, a spinal schwannoma with such manifestation is very rare. The following keywords were searched on Pubmed/MEDLINE, Index Medicus, Google scholar-spinal; schwannoma; neurofibroma; neurinoma; tumoural; intratumoural; intradural; acute; haemorrhage and haemorrhagic schwannoma. A total of 11 cases have been reported in English literature till date [1,2,5,7–13]. Ours is the second such case of intratumoural haemorrhage to be reported after a trauma.
Literature Review
The presentation is acute with rapidly progressive neurological deficits seen in most cases. This is usually due to the sudden cord compression caused by tumoural bleed, which is similar to a spinal shock. This is also the reason as to why a few cases had flaccid weakness at presentation including ours. A history of prior symptoms is uncommon in such cases. Among the 11 cases reported, 4 had prior history of backache of varying duration while remaining had no such symptoms. One patient was on anticoagulants while 1 case had a minor trauma as probable inciting event [5,7]. As noted in [Table/Fig-4], duration of symptoms ranged from 2 hours to a few days, and a slight male predominance is seen with a male: female ratio of 6:5. Individuals in the fourth to fifth decades have the highest propensity with mean age being 50 years (range 26-74 years). Isolated intratumoural haemorrhage is the most common finding while associated subdural, subarachnoid and intramedullary haemorrhage is uncommon [10,11,13]. In lieu with spinal schwannomas, the highest incidence for those with intratumoural haemorrhage is seen at the thoraco-lumbar junction, probably because of increased mobility of this segment. One case was a cervical one while other was a foramen magnum tumour with cervical extension [2,10].
Literature review of spinal schwannomas with intratumoural haemorrhage.
| Author | Age/Sex | Clinical features | Duration of symptoms | Prior history | Spinal level | Enhancement on MRI | Outcome | Follow-up (months) |
|---|
| Smith, 1985 [13] * | 74/F | Cervical myelopathy | Acute | Absent | Cervical | NP | Died | NA |
| Lee, 1992 [8] | 48/M62/M | Spastic paraplegia, urinary incontinenceFlaccid paraplegia, urinary retention | 2 days1 day | BA x 1 yearBA x 2 year | T11T12-L1 | NPNP | ExcellentExcellent | 61 |
| Uemura, 1998 [12] | 58/F | Rapid progressive weakness | Sudden onset | Absent | T12 | Peripheral | Good | NA |
| Cohen, 2000 [5] ** | 37/M | Flaccid paraplegia | 24 hours | Minor fall | T11-12 | Inhomogeneous | Fair | 12 |
| Ng, 2001 [10] *** | 43/M | UL pain, left hemiparesis | 3 days | Absent | C6-7 | Homogeneous | Excellent | 3 |
| Ciapetta, 2008 [2] | 44/F | Neck pain, myelopathy, B/L XI, UL dysesthesia | 3 days | Absent | FM-C5 | NA | Excellent | 6 |
| Tanaka, 2008 [9] # | 26/F | Severe LL weakness, urinary retention | Sudden onset | LBA x 3 years | T9-12 | Absent | Fair | 6 |
| Ichinose, 2009 [7] | 64/M | BA, severe paraparesis, urinary incontinence | Sudden onset | On anticoagulants | T12-L1 | Peripheral | Good | 3 |
| Kukreja, 2014 [11] ## | 47/M | Seizures, leg pain | Few days | Absent | L1-2 | Heterogeneous | Excellent | 3 |
| Zhang, 2015 [1] | 48/F | Severe leg pain, flaccid paraplegia | 2 hours | LBA x 1 year | T10-11 | NA | Excellent | 8 |
| Prasad, 2015 | 40/M | Flaccid paraplegia | 4 hours | Absent | C7-D3 | Absent | Fair | 2 |
F-female; NP-not performed; NA-not available; M-male; BA-backache; XI-accessory nerve; UL-upper limb; FM-foramen magnum; LL-lower limb; LBA-low backache
* This case had associated subdural hematoma
**Tumoural bleed resulted after a minor fall
***This case had associated subdural clot+ intramedullary haemorrhage
#Tumoural bleed after vaginal delivery
## There was an associated subdural clot+ intracranial SAH
All patients with acute onset neurological deficits should be imaged with MRI which is the imaging modality of choice. The signal intensities of these lesions depend upon the stages of blood; however the most common pattern seen noted is isointensity on T1 and hypointensity on T2 sequences, indicating acute haemorrhage, although other patterns may be noted [12]. Although homogeneous enhancement is seen in spinal schwannomas, those with tumoural haemorrhage usually show inhomogeneous or absent enhancement due to concomitant presence of blood products. Of the available reports, the enhancement patters were as follows-inhomogeneous in 2, homogeneous in 1, absent in 1 and peripheral enhancement in 2 cases [5,7,9–12]. In our case, there was no enhancement on imaging.
A rare subtype of schwannomas, ancient schwannoma which is most commonly seen in the head and neck, and upper extremities, is noted to have slightly higher incidence of bleed and has haemorrhages noted on histopathology. In addition, degenerative changes as mentioned above (in case illustration) are characteristically present due to aging of the tumour. But, it was ruled out in our case in view of absence of such changes on histopathology [14].
Aetiopathogenesis
Two main theories have been proposed for the occurrence of tumoural haemorrhage. According to the vascular theory, there occurs spontaneous thrombosis of hyalinised tumour vessels with resultant distal tumour necrosis and secondary bleed or alternately, vascular tumours may get obliterated by endothelial proliferation with recanalization resulting in haemorrhage. The second one, mechanical theory, postulates that movements of spine induce traction on tumour vessels resulting in haemorrhage and this usually occurs in thoraco-lumbar lesions [10,15,16]. Other postulates of haemorrhage (including subarachnoid space) include central ischemic necrosis due to tumour growth or malignant transformation with neovascularisation [15,17]. In our case, because of the absence of prior symptoms and occurrence of symptoms after a minor trauma, we speculate that the latter theory would be more suitable. This is due to its location in cervico-thoracic region wherein, probably because of the increased mobility of this segment similar to the thoraco-lumbar ones noted in most other cases, haemorrhage would have occurred.
Early surgical removal is the treatment of choice and complete removal can be achieved in almost all cases, however, with sacrifice of the originating nerve roots [7,12,13]. We agree to the opinion of Ciapetta et al., that although there is a volumetric increase in the tumour secondary to bleed, the haemorrhage per se makes the tumour softer, resulting in easy and complete removal [2]. Good outcome is noted in majority of cases as can be seen in the 10 cases where 8 out of 10 had good-to-excellent outcomes after surgery. One patient succumbed to peritonitis after surgery [13].
Conclusion
Spinal schwannoma presenting with intratumoural haemorrhage is a very rare event. It is usually seen in middle aged males and presents with acute onset neurological deficits. Tumours in thoraco-lumbar region have the highest propensity to bleed. MRI is the gold standard diagnostic modality. Emergency surgical removal is the treatment of choice and majority have good-to-excellent outcomes.
[1]. Zhang HZ, Li Y, Han Y, Wang X, She L, Yan Z, Dong L, Spontaneous acute haemorrhage of intraspinal canal cellular schwannoma with paraplegia: A case reportBr J Neurosurg 2015 29(3):425-27. [Google Scholar]
[2]. Ciappetta P, D’Urso PI, Colamaria A, Giant craniovertebral junction haemorrhagic schwannoma: case reportNeurosurgery 2008 62(5):E1166 [Google Scholar]
[3]. George B, Lot G, Neurinomas of the first two cervical nerve roots: A series of42 casesJ Neurosurg 1995 82(6):917-23. [Google Scholar]
[4]. Conti P, Pansini G, Mouchaty H, Capuano C, Conti R, Spinal neurinomas: Retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literatureSurg Neurol 2004 61(1):35-43. [Google Scholar]
[5]. Cohen ZR, Knoller N, Hadani M, Davidson B, Nass D, Ram Z, Traumatic intratumoural haemorrhage as the presenting symptom of a spinal neuroma. Case reportJ Neurosurg 2000 93(2 Suppl):327-29. [Google Scholar]
[6]. Herb E, Schwachenwald R, Nowak G, Muller H, Reusche E, Acute bleeding into a filum terminale ependymomaNeurosurg Rev 1990 13(3):243-45. [Google Scholar]
[7]. Ichinose T, Takami T, Yamamoto N, Tsuyuguchi N, Ohata K, Intratumoural haemorrhage of spinal schwannoma of the cauda equine manifesting as acute paraparesis-case reportNeurol Med Chir (Tokyo) 2009 49(6):255-57. [Google Scholar]
[8]. Lee ST, Lui TN, Acute paraplegia resulting from haemorrhage into a spinal neurofibromaParaplegia 1992 30(6):445-48. [Google Scholar]
[9]. Tanaka H, Kondo E, Kawato H, Kikukawa T, Ishihara A, Toyoda N, Spinal intradural haemorrhage due to a neurinoma in an early puerperal womanClin Neurol Neurosurg 2002 104(4):303-05. [Google Scholar]
[10]. Ng PY, Schwannoma of the cervical spine presenting with acute haemorrhageJ Clin Neurosci 2001 8(3):277-78. [Google Scholar]
[11]. Kukreja S, Ambekar S, Sharma M, Nanda A, Cauda equina schwannoma presenting with intratumoural haemorrhage and intracranial subarachnoid haemorrhageJ Neurosurg Spine 2014 21(3):357-60. [Google Scholar]
[12]. Uemura K, Matsumura A, Kobayashi E, Tomono Y, Nose T, CT and MR presentation of acute haemorrhage in a spinal schwannomaSurg Neurol 1998 50(3):219-20. [Google Scholar]
[13]. Smith RA, Spinal subdural hematoma, neurilemmoma, and acute transverse myelopathySurg Neurol 1985 23(4):367-70. [Google Scholar]
[14]. Klijanienko J, Caillaud JM, Lagacé R, Cytohistologic correlations in schwannomas (neurilemmomas), including “ancient,” cellular, and epithelioid variantsDiagn Cytopathol 2006 34(8):517-22. [Google Scholar]
[15]. Parmar H, Pang BC, Lim CC, Chng SM, Tan KK, Spinal schwannoma with acute subarachnoid haemorrhage: a diagnostic challengeAJNR Am J Neuroradiol 2004 25(5):846-50. [Google Scholar]
[16]. Cordon T, Bekar A, Yaman O, Spinal subarachnoid haemorrhage attributable to schwannoma of the cauda equinaSurg Neurol 1999 51(4):373-75. [Google Scholar]
[17]. Parmar H, Patkar D, Gadani S, Shah J, Cystic lumbar nerve sheath tumours: MRI featuresAustralas Radiol 2001 45(2):123-27. [Google Scholar]