Hence the aim of the present study was to evaluate the sensory changes in the areas of infraorbital nerve distribution following common treatment modalities used in the management of zygomatico-maxillary complex fractures and to determine whether there is any association between the rate of recovery in the infraorbital nerve function and the treatment modality used.
Materials and Methods
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000 with prior approval of the Hospital Ethical Committee, in the Department of Oral and Maxillofacial Surgery, Dr. R. Ahmed Dental College and Hospital, Kolkata, West Bengal, India from September 2012 to February 2014. History regarding the aetiology of the injury was recorded in specially prepared case sheets. Following proper clinical and radiological evaluation, 13 patients diagnosed with unilateral zygomatico-maxillary complex fractures and having sensory disturbance over the distribution of the infraorbital nerve of not more than six weeks duration were selected randomly irrespective of age, sex, caste and religion. Patients who were not medically compromised and willing to participate in the study with informed consent in black and white and who could make themselves readily available for periodic follow up were included in the study. Medically compromised patients, bilateral or infected zygomatic complex fractures, restricted ocular movements, absence of any sensory disturbances over the area supplied by infraorbital nerve on the affected side, metal allergies or foreign body sensitivity and patients not willing to participate were excluded from the study.
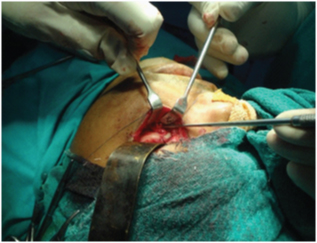
Fractures were diagnosed clinically and radiographically. Routine radiographs included paranasal sinus, submentovertex, postero-anterior views along with a Computed tomographic scan. Indications for reduction of fractures were based on the signs and symptoms including visible facial asymmetry, significant functional disturbance of mandibular movement, inadequate mouth opening, subconjunctival haemorrhage [Table/Fig-1] and depressed malar [Table/Fig-2] arch along with sensory deficit in the region of distribution of the infraorbital nerve. The clinical findings were corroborated with the radiological findings. Closed reduction was attempted, and if this resulted in a stable reduction of the fragments no further treatment was employed. Unstable reduction was considered where there was a return of a “step” at the infraorbital rim or the fronto-zygomatic suture [Table/Fig-3] or a visible lack of facial symmetry. Where reduction was unstable due to displacement between the fracture fragments, the zygomatic buttress [Table/Fig-4], fronto-zygomatic suture and the infraorbital rim [Table/Fig-5] were surgically exposed according to the need of the individual cases, reduced and immobilised with miniplates along with decompressing the nerve which released it from the soft tissue entrapment owing to trauma or organized clot at the fractured site. Patients with such unstable fracture fragments were categorized under Group A (Open Reduction with Internal Fixation) [Table/Fig-6]. In Group B, only patients where only closed method was able to achieve stable reduction were included. In Group C, patients were not subjected to either surgical or non-surgical interventions as there was no indication of reduction of fracture clinically and radiologically.
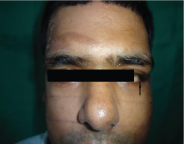
Sub-conjunctival haemorrhage
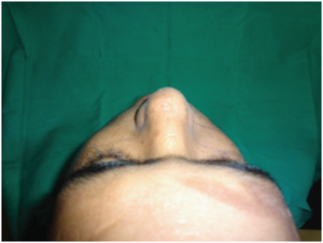
Step at the fronto-zygomatic suture
Surgical exposure of the zygomatic buttress
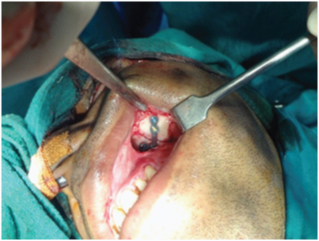
Surgical exposure of the infraorbital rim
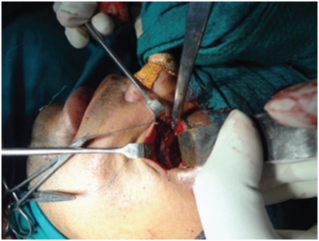
Open reduction with internal fixation
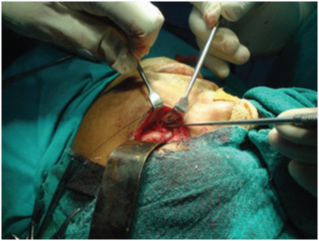
Preoperative assessment of the infraorbital nerve sensory deficit was performed in relation to the contra lateral side which acted as a control. Nerve function was assessed in terms of the parameters recorded preoperatively. During examination, the patient was asked whether abnormal sensations were experienced and the area with sensory disturbance was prepared and marked with gentian violet. The site to be stimulated was selected randomly to be as close to nerves endings as possible, but taking care that the areas stimulated are the same on both sides to avoid operator error. Patient while keeping the eyes closed indicated the area stimulated and readings were obtained in consideration to the control side first followed by the affected side on a patient assessment log. When patient pointed a diminished sensation in comparison to the control side, sensory function was defined as hypoaesthesia. Dysaesthesia was characterised by an altered quality of sensation that included an uncomfortable component. Anaesthesia was related to complete absence of sensation. Infraorbital nerve function assessment was done by mechanical threshold detection, heat threshold detection and pain threshold detection.
Mechanical Threshold Detection (A-beta fibres): The mechanical threshold detection was carried out with the help of various lengths of 7-0 nylon monofilament cut into three lengths of 3cm, 5cm and 7cm held with non-toothed forceps at one end, other end being free for purpose of testing [7]. The threads were calibrated on a laboratory balance held in a perpendicular fashion applying enough force to induce slight bending of the filament. The various lengths of the filaments were used to stimulate the area bilaterally starting with 7cm filaments and ending with 3cm [Table/Fig-7]. Each site was stimulated three times in a perpendicular fashion. Patients indicated the site with the index finger with the eyes closed as well as subjectively asserting ‘yes’ or ‘no’. More than two positive responses were taken as normal tactile sensory function.
Mechanical threshold detection by 7-0 nylon monofilament
Thermal Threshold Detection (A-delta & C-fibres): A hot water bath was used to achieve study temperatures of 32°C, 35°C and 37°C checked with a thermometer. Glass test tubes with equal volume of water were used to detect the thermal thresholds [8]. Three sites were stimulated in the test area bilaterally starting from 32°C to 37°C [Table/Fig-8]. The area was allowed to cool and relax after each temperature change to avoid false positive findings. The responses were taken as ‘yes’ when patient could feel and ‘no’ when patient could not feel. More than two positive responses were taken as normal temperature sensation.
Thermal threshold detection
Pain Threshold Detection (A-delta & C-fibres): Pain threshold was detected using a 32 gauge, one inch length acupuncture needle (Asha acupuncture needle, Filiform type no.10) and a 100 mm Visual Analog Scale (VAS) calibrated 0-10, with 0 being no pain and 10 being agonizing pain). The test was performed with the needle pushed against the patient’s skin until it slightly bent [Table/Fig-9]. The mean force exerted was calibrated on a laboratory balance and was found to be 13.35 gm. Three sites were stimulated bilaterally in a given area and the readings recorded on VAS. Any two common numerical findings were recorded as a reaction to pin prick.
Pain threshold detection by acupuncture needle
Management of The Zygomatic Complex Fracture
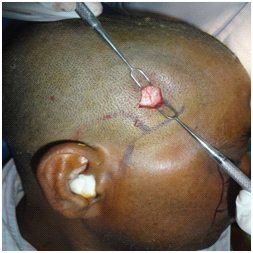
Group A: Under general anaesthesia, standard incisions were used to expose the fracture sites. Infraorbital nerve was explored [Table/Fig-10] at its exit near the infraorbital foramen. ORIF was done with 1.5mm straight mini-plate with bar, 1.5 mm C-plate with bar and 2mm. L-plate with bar for fronto-zygomatic, infraorbital and zygomatic buttress regions respectively followed by closure of the surgical wound in layers.
Exploring the infra-orbital nerve
Group B: The patients in this group were treated by closed reduction under general anaesthesia.
Two approaches used for fracture reduction were:
Gillie’s temporal approach: A 2.5cm length incision was made between the point of bifurcation of superficial temporal vessels at about 4°; to the attachment of the external ear, temporal fascia exposed [Table/Fig-11] and Rowe’s zygoma elevator passed beneath the zygomatic bone to deliver a firm upward, forward and outward elevation without undue effort [Table/Fig-12] [9].
Keen’s intra-oral approach was carried out through the same incision used to expose the zygomatico-maxillary buttress. A curved elevator was passed upward supra-periosteally to contact the infratemporal surface of zygomatic bone to exert an upward, forward and outward pressure to reduce and disimpact the fracture [10].
Incision with exposure of temporo-parietal fascia
Group C: Patients in this group were not subjected to either surgical or non-surgical interventions. Although there was sensory deficit with radiological evidence of zygomatico-maxillary complex fracture yet clinically there was no gross derangement of occlusion, facial asymmetry or difficulty in mouth opening.
Follow Up of The Patients
Evaluation of the patients were done on 1st, 3rd, 7th day, one month, [Table/Fig-13] three months [Table/Fig-14] and six months [Table/Fig-15] in a manner similar to the assessment done at the beginning of the study (Day 0). Infraorbital nerve function was assessed postoperatively and any improvement in nerve function was recorded and compared with the pre-operative assessed data. Sensory threshold was quantified for each of the modalities bilaterally. Our purpose was to find out whether there are factors which are of value in predicting the recovery of the infraorbital nerve.
1 month post-operative view
3 months post-operative view
6 months post-operative view

Results
The mean age was 30.53 years and male patients above 25 years of age were significantly more affected (male:female ratio of 5.5:1) with a definite predilection towards the right side of the face (69.2%). Road traffic accident (92.30%) was the most common cause for zygomatic complex fractures followed by occupational injuries (7.69%). There was a relationship between different lengths of 7-0 nylon monofilaments with the degree of force exerted and the outcomes of nerve injuries [Table/Fig-16]. In this study, zygomatico-maxillary complex fractures of Henderson’s classification Type II, III and V(23.06% each) were more prevalent. Seddon’s Class I type of nerve injury (53.84%) was significantly higher than Class II (38.46%) [Table/Fig-17]. Sub-conjuctival haemorrhage was most common (84.61%) followed by depressed malar arch (69.23%) and distraction at fronto-zygomatic suture (38.46%). Hypoesthesia was the most common presenting neurological symptom (76.92%) followed by dysesthesia (7.6%) and paresthesia (7.6%). Mean time for surgical intervention was 3.4 weeks (30.76%). Most of the patients were treated by closed reduction (46.2%) followed by ORIF (38.5%) while 15.4% cases were treated conservatively [Table/Fig-18].
Relationship between different lengths of 7-0 nylon monofilaments with the degree of force exerted
| Length(cm.) of 7-0 nylon monofilament | Force(gm.) |
|---|
| 7.0 | 0.0038 |
| 5.0 | 0.0080 |
| 3.0 | 0.0220 |
Distribution of Henderson’s classification of zygomatic complex fractures and Seddon’s classification of nerve injury
| Class/Type | Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type6 | Type 7 |
|---|
| Class 1 | 2 | 3 | 2 | | | | |
| Class 2 | | | 1 | 1 | 3 | | |
| Class 3 | | | | | | 1 | |
| Type of Treatment | Number | Percentage |
|---|
| Group A(ORIF) | 5 | 38.5% |
| Group B(Close Reduction) | 6 | 46.2% |
| Group C(Conservative Treatment) | 2 | 15.4% |
| Total | 13 | 100% |
For mechanical threshold detection at Day 0, proportion of patients with sensation at 3cm length of filament for Group A (60%) was significantly lower than the other two groups. It was significantly lower at 5cm and 7 cm for Group A than Group C (p<0.05). There occurred a gradual degradation in the mechanical threshold detection. However patients treated conservatively showed no change. At Day 7, patients with sensation at 3cm and 5cm for Group A (20%) was significantly lower than Group C (p<0.05). At one month patients with sensation at 3cm for Group A was significantly lower than Group C (p<0.05). However, Group C showed inability in detection of 7cm filament. At three months, patients with sensation at 3cm and 5cm for Group A (20%) was significantly lower than Group B and C (p<0.05). At six months, proportion of patients with sensation at 3cm and 5cm for all the three types of treatment was significantly higher (p<0.05). It was observed that the patients in Group A and B showed considerable improvement as some kind of intervention was done. However Group C showed deterioration in mechanical threshold detection [Table/Fig-19].
Mechanical threshold detection by 7-0 nylon mono filament at three and six months interval
| 3 Months | 6 Months |
|---|
| Sensation Appreciated | Sensation Appreciated |
|---|
| Group A |
|---|
| Length | Yes | No | Yes | No |
|---|
| No. | % | No. | % | No. | % | No. | % |
|---|
| 3cm | 4 | 80 | 1 | 20 | 5 | 100.0 | 0 | 0.0 |
| 5cm | 1 | 20 | 4 | 80 | 4 | 80.0 | 1 | 20.0 |
| 7 cm | 0 | 0.0 | 5 | 100 | 0 | 0.0 | 5 | 100.0 |
| Group B |
| 3cm | 6 | 100 | 0 | 0.0 | 6 | 100.0 | 0 | 0.0 |
| 5cm | 5 | 83.3 | 1 | 16.7 | 6 | 100.0 | 0 | 0.0 |
| 7 cm | 0 | 0.0 | 6 | 100 | 1 | 16.7 | 5 | 83.3 |
| Group C |
| 3cm | 2 | 100 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 |
| 5cm | 1 | 50 | 1 | 50.0 | 1 | 50.0 | 1 | 50.0 |
| 7 cm | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 | 2 | 100.0 |
For pain threshold detection, there was some major deterioration in the sensory function in Group A subjects postoperatively which improved with time and at the end of six months it was between score 1 and 2 on Visual Analogue Scale (VAS). Group B showed mild deterioration in the reaction to pin prick [Table/Fig-20] which improved with time but was less than Group A after six months. Interestingly Group C subjects improved with time but a deterioration was seen at the end of six months.
Pain threshold detection (with acupuncture needle)
| Pain Threshold Detection(With Accupuncture Needle) | Day0 | Day1 | Day 7 | 1Month | 3 Month | 6Month |
|---|
| Group A | 1.00±0.0 | 0.20±0.44 | 0.80±0.73 | 1.00±0.70 | 1.20±0.44 | 1.60±0.54 |
| Group B | 1.33±0.81 | 1.16±1.47 | 1.00±0.63 | 1.16±0.40 | 1.50±0.54 | 1.50±0.54 |
| Group C | 1.50±0.70 | 1.50±0.70 | 2.00±0.0 | 1.50±0.70 | 1.50±0.70 | 1.50±0.70 |
For heat threshold detection, proportion of patients with sensation at 32°C and 35° C for Group A (60%) on Day 0 was significantly lower than Group C (p<0.05). On Day seven, patients with sensation at 32°C for Group A (60%) was significantly higher but at 35°C it was significantly lower for Group A than Group C (p<0.05). Group A and B showed gradual increase in thermal threshold detection. After one and three months patients with sensation at 32°C for Group A was significantly higher than Group C (p<0.05). Thermal sensation at the end of six months gradually increased for Group A and B [Table/Fig-21]. It was observed that thermal sensation improved between 7th day post-interventional and 1-3 months, thereafter remaining constant. Maximum improvement was found in Group B (100%) followed by Group C (83.33%) and lastly in Group A (80%).
Thermal Threshold Detection (Test tube with hot water at different temperatures)
| 3 months | 6 months |
|---|
| Sensation appreciated | Sensation appreciated |
|---|
| Group A |
|---|
| temp | Yes | No | Yes | No |
|---|
| No. | % | No. | % | No. | % | No. | % |
|---|
| 320C | 2 | 40 | 3 | 60 | 3 | 60 | 2 | 40 |
| 350C | 3 | 60 | 2 | 40 | 4 | 80 | 1 | 20 |
| 370C | 5 | 100 | 0 | 0 | 5 | 100 | 0 | 0 |
| Group B |
| 320C | 2 | 33.3 | 4 | 66.7 | 4 | 66.7 | 6 | 100 |
| 350C | 6 | 100 | 0 | 0.0 | 0 | 0 | 6 | 100 |
| 370C | 6 | 100 | 0 | 0.0 | 0 | 0 | 6 | 100 |
| Group C |
| 320C | 0 | 0 | 2 | 100 | 2 | 100 | 1 | 50 |
| 350C | 1 | 50 | 1 | 50 | 1 | 50 | 0 | 0 |
| 370C | 2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
ANOVA [Table/Fig-22] showed that there was significant difference in pain score for different treatment at different time intervals (F2, 54=623.62; p<0.01). Test of proportion showed that the trend in recovery of the infraorbital nerve was relatively complete in 76.92 % cases at the end of this study, while partial recovery was observed in 23.07% cases [Table/Fig-23].
ANOVA table for pain score
| Source | Degrees of freedom | Sums of squares | Mean sum of squares | ‘F’statistic | ‘p’ |
|---|
| Between Groups | 2 | 1933.25 | 966.62 | 623.62 | <0.01 |
| Residual | 75 | 116.25 | 1.55 | - | |
| Total | 77 | 2017.35 | - | - | |
Trend in recovery of infraorbital function
| Recovery | Number | % |
|---|
| Complete | 0 | 0.00 |
| Relatively Complete | 10 | 76.92 |
| Partial/ Incomplete | 3 | 23.08 |
| Tota | 13 | 100.00 |
Discussion
The mean age of the patients was 30.53 years with a male predominance (5.5:1) and commonly affecting the right side of face. Road traffic accident was the most common aetiology. Similar findings were reported by other workers as well. Kovacs et al., reported of fractures around the 2nd and 3rd decade in males and having a ratio of 4:1 over females [11]. Chakranarayan et al., found that 53.3% fractures were on the right side [12]. Motamedi reported of road traffic accident in 30.8% cases [13]. Sensory deficit of the areas supplied by the infraorbital nerve was the most common presenting neurological symptom in our study. The infraorbital nerve may be involved in trauma to the zygomatic complex which often results in sensory disturbance of the area innervated by it [14]. Paresthesia was the most common symptom (64.3%) after zygomatico-maxillary complex fractures [15].
This study included 13 patients with unilateral zygomatico-maxillary complex fractures. According to Henderson’s Classification (1963) in this study Type IV, V and VI needed reduction with fixation while Type II and III required closed reduction and Type I was in no treatment group [16]. Vriens et al., in their study reported of Henderson’s Type I, III, IV and V type of fractures and required fixation [17]. In this study, 84.61% of the patients had sub-conjuctival ecchymosis, 69.23% patients had a depressed malar arch while 38.46% cases had radiographic distraction at fronto-zygomatic suture. Calderoni et al., found 46.2% of the patients with ecchymosis/haematoma and malar depression in 28.6% cases [18]. In accordance to Seddon’s classification of nerve injury (1943) [19] in this study, Class I (53.84%) type was significantly higher than class II (38.46%) or Class III (7.69%). Taicher et al., postulated that the nerve can be damaged through oedema, ischaemia, compression, traction and/or rupture by the bony spicules of a disrupted orbital floor and/or sharp edge of the fracture line [20]. The incidence of sensory disturbances in orbito-zygomatic complex fractures in the immediate post-trauma period varies from 24% to 94%. Our findings corroborate with the observations of Vriens [17,21] and Ellis et al., [3].
We found hypoesthesia being the primary complaint with an incidence of 76.92% while hyperesthesia, dysesthesia and paraesthesia were found in 7.6%cases each. Robinson stated that minor compression will give rise only to temporary conduction block, while more severe compression injuries causes Wallerian degeneration distal to the site of injury [22]. We found that most of the injury to the nerve was owing to compression following depression and rotation of the zygomatic complex at the fronto-zygomatic suture. So care was taken during infraorbital rim fixation and surgical intervention, so as to avoid stretching of the nerve. Sunderland’s classification (1951) incorporated the features of Seddon’s scheme [23]. Where neuropraxia or 1st degree lesions exist, return to normal sensory function occurs within one week following nerve injury, 1st degree (type 3) takes 1 to 2 months for complete recovery. A neurotmesis or 3rd, 4th or 5th degree nerve injury will show incomplete recovery of sensory function owing to severe traction or compression. Following a compression injury it is quite possible to have a deficit in mechanoception but intact nociception and thermal discrimination [24]. In our study, there were diminished mechanical, thermal and pain threshold for patients in open and closed reduction followed by gradual recovery which started after seven days with peak recovery between three to six months. In the non-surgical group there was some sensory loss after one month especially in tactile and pain detection thresholdsimilar to the observations of De Van et al., Jungell and Pedemonte [25–27]. In this study, the mechanoception recovered but not completely. The thermal receptors and nociceptors (A-delta and C) showed a recovery which was relatively complete. Thermal sensations at the end of six months gradually recovered in patients with open and closed reduction whereas a patient in the non-surgical group showed deterioration in symptoms. It was noticed that thermal sensation was present in non-surgical group or recovered after surgery within one week as in open and closed reduction patients, well before fine touch. Pain receptors recovered after one week of intervention in all the groups. Neurological deficit at the end of the study was found to be 23.07%. Benoliel et al., found that thermal and painful stimuli are thought to be transduced by thin, unmyelinated (C) and thin myelinated (A-delta) fibres [7]. Mechanical and electrical stimuli selectively activate thick myelinated fibres (A-beta). Their findings indicated that thin nociceptor fibres recover to a larger extent than the mechanoreceptors.
In this study, we observed that relatively complete recovery occurred in 76.92% of our patients. The incidence of postoperative infraorbital nerve sequelae was diminished considerably in unstable zygomatic fractures when treated by osteosynthesis with mini-plates. Ellis et al., presented an analysis of 2067 zygomatico-orbital fractures where 23% did not receive any surgical intervention, 70% cases were treated by simple elevation via temporal approach [3]. Of the 1521 patients who had unilateral zygomatico-orbital fractures, 30% required fixation. Champy et al., stated that reduction and fixation are important factors in the recovery of infraorbital nerve [28]. Jungell P studied 50 patients treated by various methods [26]. Half of the patients who did not receive any treatment had some degree of parasthesia. A 94% of the patients who received operative treatment had improvement in sensory function. Prachur Kumar et al., concluded that earlier the surgical intervention, more the recovery of the nerve injury is appreciable during the 1 and 6 months follow up period [29].
Conclusion
Our analysis showed marked improvement in the neurosensory deficit of the infraorbital nerve when some form of treatment in the form of open or closed reduction was applied which facilitated an early recovery of the nerve within a span of one to six months.