Dental implants serve as an artificial tooth roots and have been successful in preventing the physical and cosmetic consequences associated with tooth loss [1]. The long term survival of dental implants is evaluated by the amount of crestal bone loss around the implant [2]. This peri implant crestal bone level and peri implant bone remodelling depends upon location of Implant Abutment Junction (IAJ) in relation to bone crest and the amount of soft tissue coverage [3]. Numerous studies [4–6] have been done describing methods to prevent crestal bone loss. The amount of bone loss expected from implant during first year must be less than 2 mm, apical to IAJ. After first year it is expected that bone loss will be 0.2 mm annually [7]. Recording the bone level right from placement could identify role of different factors at various stages since crestal bone loss is a cumulative effect of myriad of factors [8]. Although there are many studies on the effect of placement depth after implant loading but there is a lack of sufficient number of studies on factors affecting bone loss before loading [9]. Such information could ease the job of clinician confused in judging the decision on placement depth.
Therefore this study was planned to compare the crestal bone loss around implant placed at subcrestal level and equicrestal level before prosthetic loading that could highlight the effect of placement depth alone on crestal bone loss. The null hypothesis considered here was that there is no difference in crestal bone loss in implants placed at equicrestal and subcrestal levels. Research question proposed was “Do sub crestal placement of implants help to reduce the amount of crestal bone loss?”
Materials and Methods
After obtaining institutional research and ethical committee approval (IGIDSIRB2014PROS01PGNBDP) the present study was started in the Department of Prosthodontics, Crown And Bridge and Implantology, Indira Gandhi Institute Of Dental Sciences, Puducherry during the period from 2012 to 2014. This prospective clinical trial portrays the practical clinical scenario of the implants placed in two different placement depths and meticulously followed up after six months, before prosthetic loading [Table/Fig-1]. A total of 24 implants were planned and were divided into two groups as Group E (12 nos) equicrestal placement (placed at the level of crest) and Group S (12 nos) sub crestal placement (placed 1 mm below the level of crest). The sample size of 24 was arrived by using mean and mean difference values, from previous studies and computing the data using open EPI calculator {open epi version 3.03 (2008), Atlanta, GA, USA}. The p-value was set at 0.05 with a power of 80% and confidence interval of 95%. The age group of the patients included in the study was between 23 to 45 years. The details of the treatment procedures were clearly explained and informed consent was obtained in Tamil/English from patients participating in this clinical trial.
Flow chart representing overview of methodology
After a thorough clinical examination, evaluation of diagnostic casts and scrutinizing according to the inclusion and exclusion criteria, all the patients who visited the outpatient department during the period from June 2012 to July 2013 for implant placement were recruited for the study. The patients who were motivated for implant treatment, fairly good oral hygiene, well healed edentulous ridges, periodontally sound neighbouring teeth, adequate inter arch space (10 mm), non-smokers, and patients with no systemic disturbances and bone metabolic disorders were included in the study. Patients having systemic disease (diabetes, hypertension, osteoporosis, cardiac complications), chronic smokers, pregnant mothers, patients having poor oral hygiene, parafunctional habits, supra eruption of opposing teeth, periodontitis were excluded from the study. Investigations were carried in all patients to rule out systemic disorders.
Height and width of the implants were chosen depending on the clinical situations. The available bone quantity and quality in the edentulous region was evaluated [Table/Fig-2]. Standard two stage surgical protocol was followed for all the patients [8]. Infiltration of local anaesthesia (Lox 2%, Neon pharmaceuticals, Mumbai, India) was done on the surgical site and the numbness was confirmed [Table/Fig-3]. Mid-crestal incision was made along the ridge and full thickness flap was elevated buccally and lingually to the level mucogingival junction, exposing the alveolar ridge of the implant site [Table/Fig-4]. A crestotome was used to recontour the bone to provide a reasonably flat bed for the implant site whenever needed. Once the implant site is prepared a surgical guide or stent was placed intraorally, and implant site was marked to the depth of 1-2 mm using punch drill or round bur, drilling through the cortical bone. Drill was irrigated copiously with saline internally to prevent over heating of the bone. Recipient site was verified for position and angulations with paralleling tool. Subsequent drilling was done in sequence to widen the site to accommodate the selected size of implant. Stoppers were attached to the drills to control the depth of placement of the implants.
Estimation of ridge width using the bone calliper in 36,37 region
Preoperative photograph of the surgical site after crestal incision
Surgical site preparation with twist drill
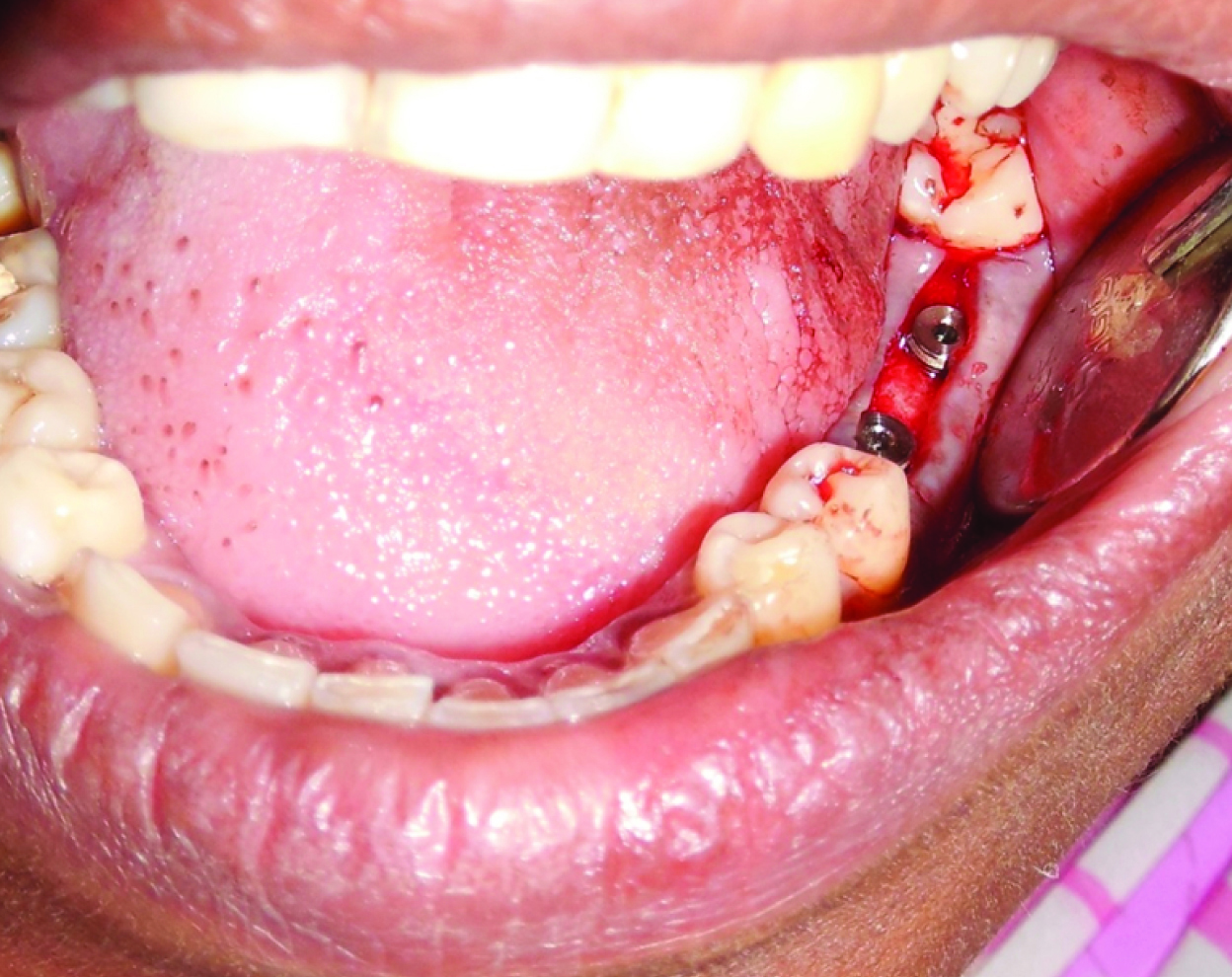
Once the desired size of the recipient site was achieved, implant, ADINT ouareg implants {Adin, Co., Israel} was placed into the recipient site using torque wrench {Adin, Co., Israel}. Implants were torqued till acceptable level of primary stability was obtained [Table/Fig-5]. Cover screw was placed and suturing was done using simple interrupted sutures {Ethicon, non absorbable surgical suture, Johnson and Johnson Ltd, Baddi, H.P, India) to keep the bleeding edges of the flap together and closure without tension [Table/Fig-6].
Placement of implant into the prepared surgical site
Approximation of flaps and placement of sutures.
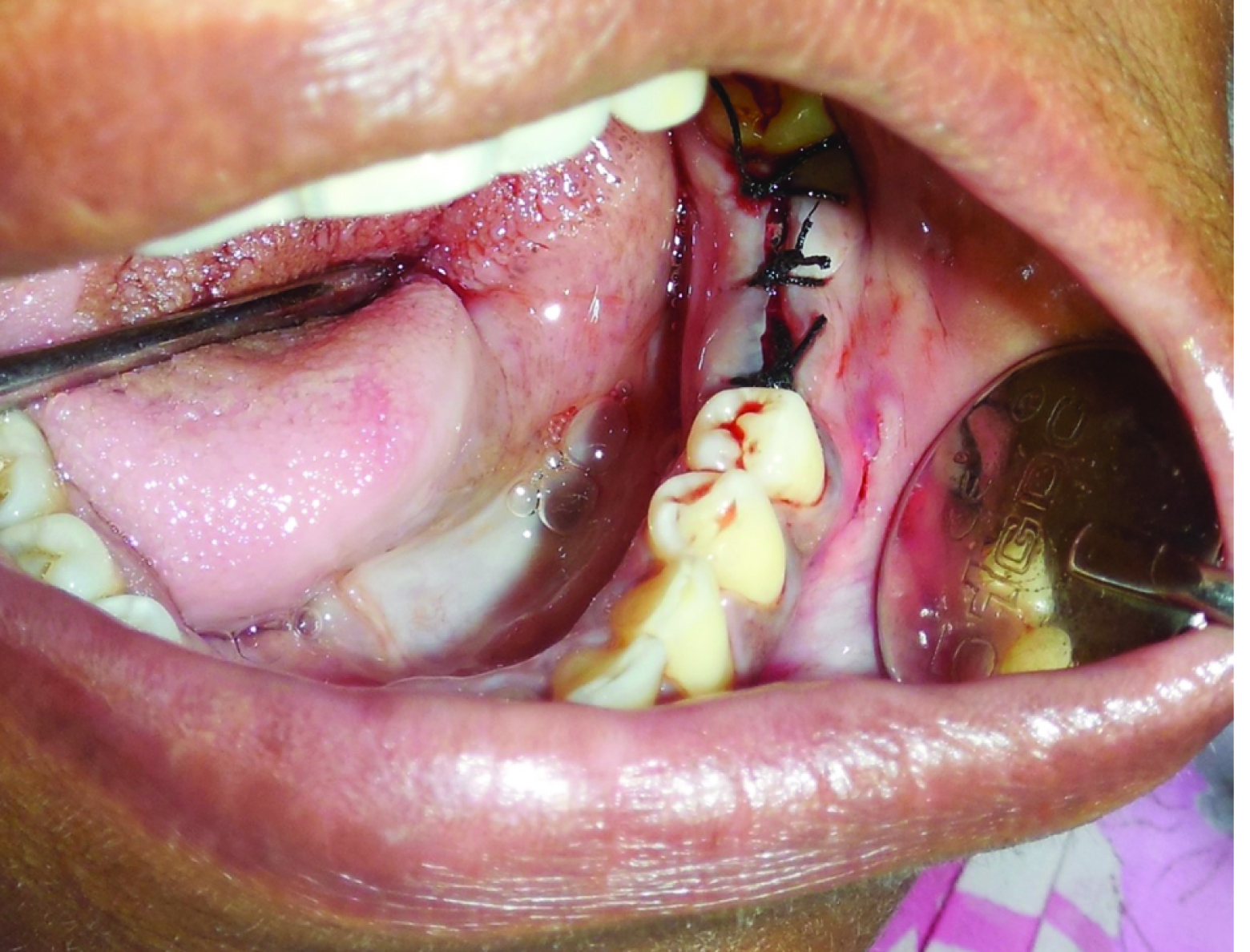
Postoperative antibiotics (Amoxycillin 500mg three times a day for three days) and analgesics (Aceclofenac100mg and paracetamol 500mg combination) were prescribed for all the patients. Extra oral cold ice packs were advised intermittently for two hours. Chlorhexidine gluconate mouthrinses (twice daily) was advised from the second day onwards. Patient was advised to take liquid or semisoft diet for two days and was advised strictly to refrain from tobacco and alcohol. Wound healing was evaluated and suture removal was done after a week.
Patients were recalled for second stage surgery after 3-6months [10,11]. A conservative incision was made on the soft tissue over the cover screw and healing abutment was placed. Digital Radio Visiography (RVG) images were taken after six months of implant placement for evaluation of marginal bone levels. Image measurement software by KLONK (KLONK 13.2.2.12 Klonks. M.B.A Norregade Denmark) was used to measure the distance in the radiographic image [Table/Fig-7]. Calibrated measurements were conducted starting from the first thread of the implant [12]. Measurements for mesial and distal portion were made from first thread to the apical and coronal portion of crestal bone respectively and mean of the coronal and apical measurements were calculated respectively. The mean of the mean mesial and mean distal values was calculated for each implant site and the resulting values were analysed.
Measurement of crestal bone loss using software (KLONK).
Statistical Analysis
The collected data was checked for normality and found to be normal and hence parametric tests were used. Unpaired t-test was used to analyse statistical significance between the groups. The p-value was set at 0.05 with a power of 80% and confidence interval of 95%. Statistical analysis was done using Graph Pad open software (Prism 6 Graph pad Inc La Jole USA).
Results
This study was done to evaluate the effect of depth of placement of dental implants in relation to the crestal bone, to observe their effect on crestal bone remodelling and its significance in prevention of crestal bone loss during six months follow up before prosthetic loading.
The collected data was tabulated. Both group E and group S were followed up for a period of six months. On six months follow up group E showed crestal bone levels (1.31±1.04 mm and 0.68±1.08 mm on mesial and distal surfaces respectively) that was apical to group S (0.49±0.49 mm and 0.025±6.06 mm on mesial and distal surfaces respectively). Even after six months, crestal bone level in group S was coronal to that of group E. Unpaired t-test was done to analyse the significance of the data obtained for bone level between both the groups [Table/Fig-8]. The p-value of crestal bone level for six months readings on mesial surface and distal surface between both the groups was 0.12 (p>0.05) and 0.07 (p>0.05) respectively which was statistically not significant.
Estimation of difference in bone levels between two groups using Unpaired t-test.
| Implantsurface | Follow upperiod | Groups | N | Mean± S.D(IN mm) | p | Significance |
|---|
| Mesial | 6 months | Equicrestal | 12 | 1.31±1.04 | 0.12 | Not sig |
| Sub crestal | 12 | 0.49±0.49 |
| Distal | 6 months | Equicrestal | 12 | 0.68±1.08 | 0.07 | Not sig |
| Sub crestal | 12 | 0.025±6.06 |
On comparing the bone loss between groups after six months follow up, group E recorded with mean bone loss (0.2183±0.2874 mm) that was less than that of group S (0.4917±0.4981) on the mesial side. Distal surface also showed group S (0.683±1.081) had more bone loss than group E (1.025±0.606 mm). Thus group S had more bone loss compared to that of group E. Bone loss between groups on mesial and distal sides [Table/Fig-9,10] were 0.11(p>0.05) and 0.35 (p>0.05) respectively and were not statistically significant. Based on the results, null hypothesis of the study was accepted that both subcrestal and equicrestal placement of implants does not have much difference in crestal bone loss. This was substantiated by statistical analysis showing significant results.
Estimation of amount of bone loss between two groups on mesial side using Unpaired t-test.
| Group | N | Mean± S.D | p | Significance | t-value |
|---|
| Equicrestal | 12 | 0.2183±0.2874 | 0.1139 | Not Sig | 1.6466 |
| Sub crestal | 12 | 0.4917±0.4981 |
Estimation of amount of bone loss between two groups on distal side using Unpaired t-test.
| Group | N | Mean± S.D | p | Significance | t-value |
|---|
| Equicrestal | 12 | 0.683±1.081 | 0.35 | Not Sig | 0.9549 |
| Sub crestal | 12 | 1.025±0.606 |
Discussion
Individual effect of placement depths in relation to crestal bone is crucial to decide on the choice of placement depth (equi or subcrestal) during the surgical phase of treatment. Literature review shows very few studies [9] evaluating the factors affecting crestal bone loss dental implants before loading. Many procedural and biomechanical factors [13–15] like implant design, micro movement, second stage surgery may lead to disruption of junctional epithelium leading to more crestal bone resorption after loading [16]. Therefore this study was planned to evaluate the amount of bone loss before loading, to study the influence of placement depth in isolation with relation to crestal bone.
In successfully osseo-integrated implants the process of initial breakdown begins at the crestal region. In our study the mean crestal bone level in mandible was more coronal than that of maxilla after six months follow up which may be attributed to thin buccal cortical plate as in a study done by Nicholas Elian [17]. Mean crestal bone level for male and female subjects in our study did not reveal any significant difference.
The results of our study showed nearly equal amount of bone loss on both equicrestal and subcrestal group after six months (before prosthetic loading) which is similar to study conducted by Richard Koh [18]. Radiographic and Histological studies [2,16] also revealed that crestal bone loss is seen in all two-piece implants and is dependent on design of implant rather than the placement technique. However, our results are contradicted by studies done by Fickl et al., Herman et al., Pontes et al., and Singh et al., [3,16,19,20] wherein the subcrestal group resulted with significantly less crestal bone loss than equicrestal group after a period of six months after loading. Sunitha et al., also found that dental implant placement below bone crest levels has been advocated to decrease the risk of exposure of shoulder or the prosthetic component margin [21]. Prasad et al., concluded from his study that crestal bone loss decreases as the distance between IAJ and the crestal bone increases [2].
From our study, it was found that there was no significant difference between equicrestal and sub crestal implant placement in crestal bone loss before loading, choice of depth of placement should be dictated more by clinical conditions (available amount of depth of bone, the existent bone quality, location of interdental papilla, type of prosthetic component) which have to be considered before deciding placement depths, since it alone does not influence bone remodelling positively.
Limitation
The limitation of our study is that radiographic measurements were done using Digital Periapical Radiographs (RVG) which gave only two dimensional data. Precise CT scan (3 dimensional) analysis of bone levels could have given more accurate and undistorted interpretations, however it carries risk of radiation overexposure. Also, clinical scenario around the implant placed in our study was not the same as there were difference in quality and quantity of the bone.
The results of our study can be corroborated with increased sample size and conducting randomized control trials in future. Prosthetic loading is on progress for these patients. Bone levels after loading will be recorded, interpreted as follow up study in the near future.
Conclusion
Within the limitations of the study it can be concluded that the implants placed at subcrestal and equicrestal levels do not show difference in crestal bone loss before prosthetic loading. Therefore the choice of placement depths can be based on synergestic action of other factors required for successful implant treatment.