The major role of micro-organisms in the initiation and perpetuation of peri-radicular lesions has been very well established. On clash of microorganisms from the infected pulp with the host’s immune system, slow necrosis of entire pulp results and leads to development of peri-radicular diseases. An infected root canal system either due to caries exposure or trauma cannot be eliminated by the host defense mechanisms alone or in combination with systemic antibiotic therapy. Therefore there arises a need to supply therapy in local way, along with mechanical preparation which has been referred to as chemo-mechanical preparation, as both components are necessary for successful procedural outcomes and are generally performed together [1].
Historically, countless compounds have been suggested as root canal irrigants. Sodium hypochlorite (NaOCl) is the most frequently used endodontic irrigating solution however, it is known to have a cytotoxic effect in the periapical area on extrusion. Chlorhexidine gluconate (CHX) because of its relatively low toxicity, substantivity and antibacterial properties against gram negative and gram positive bacterai as well as yeast has been widely used in the dentistry. Despite the advantages of CHX, its activity is pH dependent and is greatly reduced in the presence of organic matter [2].
Antibiotics were first discovered in 1928, although first reported local use of an antibiotic in endodontics was in 1951 by Grossman [3]. In dentistry including endodontics, antibiotics may be administered systemically (orally or parenterally) and applied locally (as an endodontic irrigant or endodontic medicament) [4]. High concentration has been used to efficiently eliminate residual bacteria as penetration of cytotoxic vapour forming medicaments such as formaldehyde into the periodontium have undesirable consequences by getting distributed widely in the body [5]. Minimizing the contact time of antibiotic within the root canal might be able to end this draw back, thereby establishing a need for an irrigant. Various antibiotics have been used which includes tetracycline, metronidazole, ornidazole, ciprofloxacin and minocycline.
Even though there are studies available regarding the antibacterial efficacy of individual antibiotics as an endodontic irrigant, there are no studies existing in the literature regarding the use and efficacy of the combination of tetracycline, ciprofloxacin and ornidazole as an endodontic irrigant but some studies have used antibiotics in paste form as an intracanal medicament [6–8]. Hence, this study was undertaken to suggest triple antibiotic solution containing tetracycline, ornidazole and ciprofloxacin as a new endodontic irrigant that may possess superior antibacterial activity in comparison with chlorhexidine solution.
Materials and Methods
This study was conducted between December 2012 to September 2014 in the Department of Pedodontics and Preventive Dentistry, Teeranthkar Mahaveer Dental College and Research Center, subjects diagnosed clinically as study group and with age range between 12-15 years, irrespective of sex and socio-economic status (SES) after a written informed consent has been signed. All the selected teeth were single rooted, asymptomatic, fractured, non-vital and necrotic teeth and patients were healthy and had not received antibiotic treatment during the previous months were included in the study. Teeth associated with intraoral or extra oral sinus, presence of abscess/soft tissue swelling in relation to the involved tooth were excluded from the study.
Three groups of 20 teeth each were included, depending upon the type of irrigant:
Group 1– Normal saline (Pentagon Labs Limited, Dewas (M.P), India).
Group 2– Chlorhexidine solution (Indoco remedies Ltd., Aurangabad, India).
Group 3– Triple antibiotic solution (Indigenously prepared).
Preparation of Triple Antibiotic Solution
All the raw ingredients [Table/Fig-1] were weighed with the help of electronic balance as per the formulation sheet [Table/Fig-1]. Sucrose powder was sieved through the # 20 micron mesh and collected in a separate container. All the other ingredients were also sieved through the mesh and collected in a separate container. After which the materials were mixed with sucrose powder and triturated using a glass mortar and pestle to avoid formation of any lumps. The mixture was collected in a polythene bag and packed for further use.
Raw materials required for Triple antibiotic irrigating solution preparation.
| Drugs | Amount for 100ml | Function |
|---|
| Tetracycline | Equivalent to 1g | API* |
| Ornidazole | Equivalent to 1g | API* |
| Ciprofloxacin hydrochloride % | Equivalent to 1g | API* |
| Sodium benzoate | 0.1g | Preservative |
| Sodium chloride | 0.5g | Tonicity adjuster |
| Sodium citrate | 2.9g | Buffer base |
| Citric acid anhydrous | 2g | Buffer base |
| Colloidal silicon dioxide | 0.5g | Stabilizer |
| Xanthan gum | 0.5g | Viscosity enhancer |
| Aspartame | 0.1g | Sweetner |
| Sucrose powder | qs** to 12g | Syrup base |
*Active pharmaceutical ingredient
* * Quantity sufficient
Method of Preparation of Triple Antibiotic Irrigating Solution
A pouch, containing 12 g of powder formulation, was dissolved in 80 ml of distilled water and finally the volume was adjusted to 100 ml with distilled water. The prepared formulation was stored in closed container and was used within 24 hours of reconstitution.
For each tooth, two samples were collected in order to assess the level of total colony forming units.
Sample A – pre-irrigation i.e. just after pulp extirpation and before irrigation.
Sample B – post-irrigation i.e. after irrigation.
Specimen Collection Procedure
The procedure was performed under local anaesthesia with 2% lignocaine hydrochloride containing adrenaline at a concentration of 1:2,00,000. The involved tooth was isolated and the surrounding area of tooth, clamp and rubber dam was disinfected using povidine iodine solution. An access cavity preparation was performed by employing sterile burs. After initial entry to the pulp space, the root canal was minimally instrumented with K file and the pulp was extirpated with sterile broach without using any irrigant. A sterile paper point was introduced into the full length of the canal and retained in position for 60 seconds for microbial sampling [9]. Sample A was obtained with a paper point’s length and diameter compatible with that of the root canal.
The paper point was removed from the root canal and was immediately placed in a sterile container containing normal saline and transferred to microbiology laboratory, Teerthanker Mahaveer Medical College, Moradabad. The canal was irrigated with the irrigant allotted to that particular group. The irrigant remained in contact within the canal for five minutes. Post-irrigation: Sample B was obtained in the similar manner as described earlier. Consequently a sterile cotton pellet was placed at the canal entrance and the root canal was left empty and temporarily sealed with intermediate restorative material. All the microbiological samples that were collected were then microbiologically processed to determine the viable colony forming units.
Microbiology Laboratory Procedure
In the microbiology lab, all the samples were incubated in the incubator at 370C for 24 hours. After 24 hours, each sample was inoculated on defibrinated sheep blood agar with the help of sterile inoculating loop of 0.04 mm diameter. Each plate was incubated aerobically at 370C in the incubator. After 48 hours the growth was evaluated and the total colony forming units were counted using a colony counter. The count per ml was recorded and multiplied with the dilution factor [10]. The viable organisms were counted as Colony Forming Units (CFU) per ml.

All the values of CFU were converted to LOG1010 for the ease of comparison and were carried out using Microsoft excel sheet (2010).
Statistical Analysis
Statistical analysis was carried out using SPSS version 17 for windows program. Kruskal-Wallis Test was used to compare the mean CFU of Group 1 (saline), Group 2 (chlorhexidine solution), Group 3 (Triple antibiotic irrigating solution) of Sample A and Sample B. Wilcoxson signed rank test was used for intra group comparison of mean CFU of Sample A (pre-irrigation) and Sample B (post-irrigation). Mann-whitney test was used for intergroup comparison of mean CFU.
Results
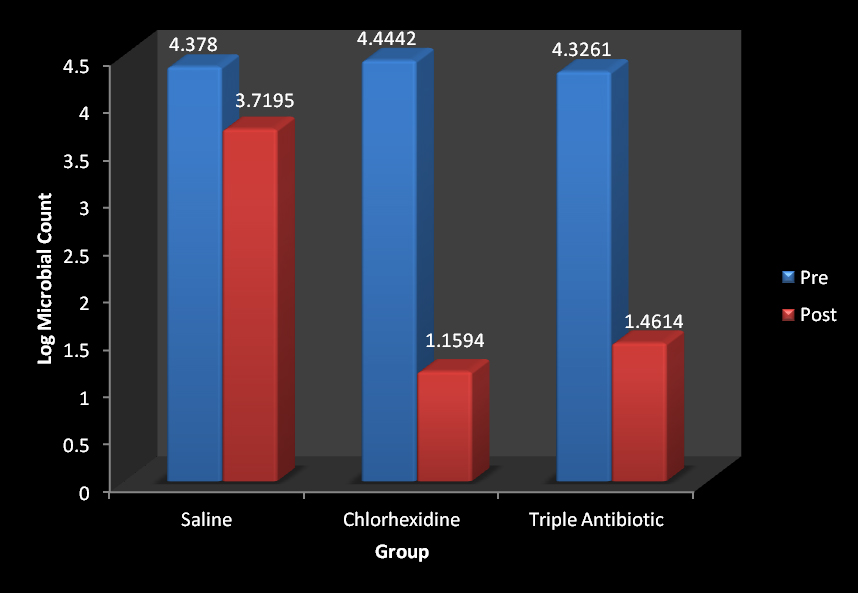
[Table/Fig-2] shows the descriptive statistics of three irrigants pertaining to pre and post-irrigation (CFU) values i.e. mean LOG10(CFU) and Std. Deviation of Group 1 (Saline), Group 2 (Chlorhexidine solution) and Group 3 (Triple Antibiotic Irrigating solution). Results suggested that statistically significant difference was observed in the mean LOG10(CFU) between the three groups in post-irrigation sample (p–0.001) [Table/Fig-3]. The intra group comparison suggested stastically significant difference between the samples of Group 1 (Saline) (p≤0.01), Group 2 (Chlorhexidine solution) (p≤ 0.001) and Group 3 (Triple antibiotic irrigating solution) (p 0.001) [Table/Fig-4]. The inter group comparison showed stastically significant result between Group 1 and Group 2 (p≤ 0.001). Group 1 and Group 3 (p<0.001) [Table/Fig-5,6]. The greatest percentage decrease was obtained in samples treated with Group 2 (Chlorhexidine solution) i.e.73.91. The triple antibiotic irrigating solution group i.e. Group 3 showed percentage decrease of 66.22 followed by Group 1 (Saline) 15.04 [Table/Fig-7].
Descriptive statistics of three irrigants pertaining to pre and post-irrigation (CFU) values i.e. mean LOG10(CFU)and Std. Deviation of Group 1 (Saline), Group 2 (Chlorhexidine solution) and Group 3 (Triple Antibiotic Irrigating solution)
| | N | Mean | Std. deviation | Std. error |
|---|
| Log Microbial count pre | saline | 20 | 4.3780 | .86878 | .19427 |
| CHX | 20 | 4.4442 | .73100 | .16346 |
| TAS | 20 | 4.3261 | .38652 | .08643 |
| Total | 60 | 4.3828 | .68238 | .08809 |
| Log Microbial count post | Saline | 20 | 3.7195 | 1.09320 | .24445 |
| CHX | 20 | 1.1594 | 1.83132 | .40949 |
| TAS | 20 | 1.4614 | 1.85384 | .41453 |
| Total | 60 | 2.1134 | 1.97451 | .25491 |
Comparison between three groups Group 1 (Saline), Group 2 (Chlorhexidine solution), Group 3 (Triple antibiotic irrigating solution) of Sample A (pre-irrigation) and Sample B (post-irrigation) using Kruskal-Wallis Test
| Log Microbial Count Pre | Log Microbial Count Post |
|---|
| Chi-Square | .753 | 19.952 |
| df | 2 | 2 |
| p-value | .686# | <0.001*** |
*** Statistically Significant
# Not significant
Intra group comparison of LOG10 (CFU) of sample A (Pre- irrigation) and sample B (post-irrigation) for Group 1 (Saline), Group 2 (Chlorhexidine solution) and Group 3 (Triple antibiotic irrigating solution) using Wilcoxson signed rank test
| Log Microbial CountPost - Log MicrobialCount Pre (Group 1) | Log Microbial CountPost - Log MicrobialCount Pre (Group 2) | Log Microbial CountPost - Log MicrobialCount Pr(Group 3) |
|---|
| Z | -3.071 | -3.922 | -3.922 |
| p-vale | .002* | <0.001*** | <0.001*** |
* Significant
*** Statistically significant
Inter group comparison of LOG10 (CFU) of sample A (Pre- irrigation) and sample B (post-irrigation) for Group 1 (Saline) and Group 2 (Chlorhexidine solution) using Mann-Whitney test
| Log Microbial CountPre (Group 1/2) | Log Microbial CountPost(Group 1/2) | Log Microbial CountPre (Group1/3) | Log Microbial CountPost (Group 1/3) | Log Microbial CountPre (Group 2/3) | Log Microbial CountPost (Group 2/3) |
|---|
| Mann-Whitney U | 187.500 | 66.000 | 181.500 | 63.500 | 168.000 | 189.500 |
| Wilcoxon W | 397.500 | 276.000 | 391.500 | 273.500 | 378.000 | 399.500 |
| Z | -.340 | -3.746 | -.502 | -3.774 | -.868 | -.334 |
| p-value | .734# | <0.001*** | .616# | <0.001*** | .385# | .739# |
# Not significant
*** Statistically significant
Mean comparison of LOG10 (CFU) of sample A (Pre-irrigation) and sample B (post-irrigation) for Group 1 (Saline), Group 2 (Chlorhexidine solution) and Group 3 (Triple antibiotic irrigating solution)
Percentage decrease in LOG10(CFU) for Group 1 (Saline), Group 2 (Chlorhexidine solution), and Group 3 (Triple antibiotic irrigating solution)
| Group | % decrease in count |
|---|
| Saline | 15.04 |
| Chlorhexidine | 73.91 |
| Triple Antibiotic | 66.22 |
Discussion
Cleaning and complete debridement of the root canal is one of important steps in the root canal treatment. Root canal irrigants have been researched often for innovative means, to end up with an ideal irrigating solution. Two broad categories were desgined for antimicrobial agents: conventional antiseptics and chemotherapeutics [11]. Conventional antiseptics included groups like alcohols, phenolic compounds, heavy metal salts, cationic detergents–quaternary ammonium compounds and halogens–hypochlorite, and iodine; chemotherapeutics includes antibiotics [11].
Chlorhexidine has been first established by Parson et al., as an antimicrobial [12]. Various concentrations which have been used of chlorhexidine for microbial growth reduction are 2% 1%, 0.2%, 0.12%. In a study carried out by Siqueira et al., effect of 2.5% NaOCl and 0.12% CHX against cultivable bacteria in teeth with apical periodontitis infected root canal systems were found to be comparable [13].
Normal saline does not result in negative cultures in a single visit thereby emphasizing the significance of an antibacterial agent [14]. Byström and Sundqvist studied the presence of bacteria in 17 single-rooted teeth with peri-apical lesions, which were irrigated with saline solution during instrumentation [15]. Mechanical manual instrumentation reduced the number of bacteria from 104–106 bacterial cells to 102–103 fewer bacterial cells. Bacteria were not detected from the root canals of eight teeth but bacteria persisted in seven teeth despite treatment on five successive occasions. However, when an antimicrobial irrigant, specifically 0.5% sodium hypochlorite, was used in place of saline, the antibacterial effect was much more effective, with no recoverable bacteria in 12 out of 15 teeth after five appointments. Neutral irrigants such as saline are not able to adequately debride canals to be free of pulp tissue debris, or bacteria. Thus normal saline has been used as a control in this study.
Various studies on triple antibiotic paste have demonstrated successful elimination of microbial pathogens [6–8,16]. Windley et al., assessed the efficacy of a triple antibiotic paste in the disinfection of immature dog teeth with apical periodontitis [16]. It was found there was significant reduction in bacteria, cultured from infected immature dog teeth, following the irrigation and antibiotic paste protocol used in this study.
In the present study triple antibiotic solution has been formulated with a composition of 1% Ornidazole, 1% Ciprofloxacin and 1% Tetracycline in 100 ml of water following, the success rate of triple antibiotic paste. In this study, 60 single rooted non vital teeth were selected.
This study was performed for evaluating the antimicrobial effect of three irrigants namely sterile saline, chlorhexidine solution and triple antibiotic irrigating solution by aerobic culture method. The root canal infection is poly microbial in nature that contains both aerobic and anaerobic microorganisms. Vianna et al., collected samples with paper points pooled in a sterile tube inoculated sample on media and incubated for both aerobically (370C, air) for 24 and 48 hours and anaerobically 370C for 7 days [17]. After incubation, the total CFU were counted using a stereomicroscope at 16X magnification. In the present study the antimicrobial activity was evaluated by aerobically incubating the microbiological samples for 48 hours. Pre-irrigation and post-irrigation sample were obtained to evaluate the role of irrigant in reducing the microbial flora present in the root canal. No instrumentation of the canal was performed so as to eliminate the role of mechanical action from the study. Devi et al., demonstrated in a similar study where 40 teeth were divided into four groups of irrigating solution [10]. One sample was recorded before irrigation and another sample was recorded post-irrigation. This study supports the study methodology carried out by Devi et al., where main action of irrigation was by flushing the root canal post extirpation of pulp and establishing antimicrobial environment against the microbes [10]. The collected microbiological samples were incubated aerobically at 370C for 24 hours and inoculated with inoculating loop of 0.04 mm diameter on blood agar media. Devi et al., streaked pre and post-irrigation sample on separate chocolate agar using a calibration loop of 0.04mm diameter that hold 0.01 ml of Robertson’s media [10]. The plate was incubated anaerobically at 370C for 72 hours and the no. of colonies were counted. Another method ‘Miles and Mishra serial dilution method’ which was observed in a study conducted by Giardino et al., [18].
This study was performed for evaluating the antimicrobial effect of three irrigants namely sterile saline, chlorhexidine solution and triple antibiotic irrigating solution by aerobic culture method. On intra group comparison all three seemed to have statistically very high reduction in microbial load. This is consistent with the findings of Akpata in 1976 who observed a significant reduction in the total viable count of microorganisms using saline as the irrigant [19]. Normal saline has the ability to remove debris from the root canal rather than having antimicrobial property. There is no statistically significant difference between chlorhexidine and triple antibiotic irrigating solution in its action. Other study by Ordinola-zapata et al., reported triple antibiotic paste better than 2% chlorhexidine and calcium hydroxide [20].
Limitation
This study was not carried on individual microorganisms since the micro-flora of the root canal is mixed in nature. The results obtained from individual micro-organisms may vary from those mixed in nature. Hence, this study where only the antibacterial effectiveness has been evaluated by incubating sample aerobically, could be further extended by evaluating the antibacterial efficacy against anaerobic micro-organisms as well individual micro-organisms. Further studies are also recommended to evaluate the physiochemical properties of the triple antibiotic irrigating solution to improvise its efficacy invivo.
Conclusion
The triple antibiotic irrigating solution used in the present study may able to niche a suitable place for itself in dentistry as it may provide complete antibacterial environment in the infected root canal. Based on the results of this it can be concluded that triple antibiotic irrigating solution has similar antibacterial activity with that of chlorhexidine solution in eliminating the aerobic microbial flora from infected root canals. Even though sterile normal saline possesses no antibacterial property but was effective in reducing the microbial load due to its flushing action.
*** Statistically Significant
# Not significant
* Significant
*** Statistically significant
# Not significant
*** Statistically significant