Separate occurrence of thyroid and parathyroid carcinoma in patients is extremely rare, and to the best of our knowledge, only 7 patients with documented parathyroid and papillary thyroid carcinomas have been described formerly in published reports. We report a patient with an extremely unusual clinical presentation of Hürthle cell carcinoma in thyroid and parathyroid carcinoma. The patient displayed a rare presentation of life-threatening hypercalcaemia after total para-thyroidectomy and failed to respond to standard therapy. Our review of available literature yielded insufficient evidence in managing such. When a patient with thyroid cancer is diagnosed, checking for serum calcium is advised. This is considered a useful method for detecting possible incidental parathyroid lesion and screening the probable concealed parathyroid pathology.
Hypercalcaemia, Hashimoto’s thyroiditis, Primary hyperparathyroidism, Thyroid nodule
Case Report
A 21-year-old man with no significant past medical history was referred to our hospital and physical examination showed a palpable right anterior neck mass and laboratory evidence revealed hypothyroidism. Thyroid disorder and malignancy was not mentioned in his family history. He denied any past history of neck irradiation. His fine-needle aspiration (FNA) biopsy was characteristic for Hashimoto’s thyroiditis. Therefore, levothyroxine was prescribed for him and he was discharged.
After 7 months treatment, the patient was referred to our endocrine clinic again with complaints of a progressive mass. Examination of the thyroid gland demonstrated a 6×4 mm mass in the right lobe, suggestive of a tumour. Second FNA revealed follicular malignancy and right lobectomy was done. The histopathology sections revealed thyroid tissue with thyroid follicles in isthmus area. Pleomorphic, low mitotic hurthle cells and clear cells filled right lobe by a solid pattern separated by scattered tiny fibrous tissue. Capsular and vascular invasion are also presented. These findings were consistent with hurthle cell carcinoma. Hence patient was prepared for another surgery. Left thyroid lobectomy, ischmectomy and cervical lymph node dissection were performed. Postoperation, pathologic report of nodular goiter was without Hürthle cell carcinoma in the thyroid, and no lymph node metastasis was identified among lymph nodes dissected through the second surgery. The patient presented hypocalcaemic symptoms after both surgeries. Although these symptoms were worse after the second surgery (Postoperation concentration of Ca and P were 6.5 and 5.5 mg/dl, respectively) and included: Numbness and tingling sensations in the perioral area and Seizure. These developed hypocalcaemic symptoms required treatment with Interavenous (IV) calcium therapy.
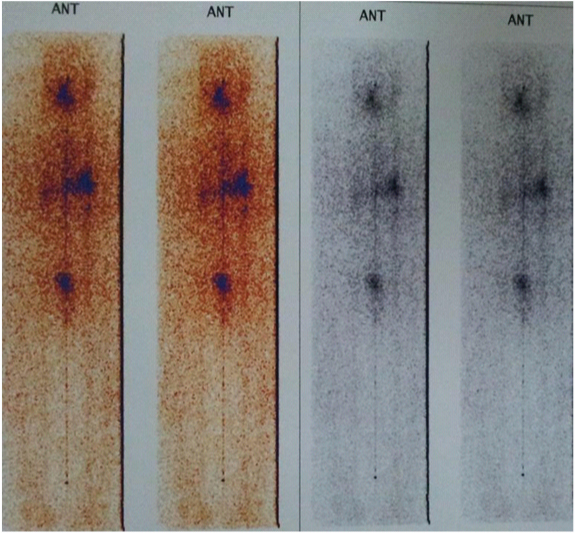
About 3 week later, the patient received radioiodine and a post-ablation whole-body scan with 131I revealed no remnant thyroid tissue [Table/Fig-1]. The patient was followed up over 2 years after completion of therapy. During this period, the patient was known as Hypoparathyroid and he was treated with levothyroxine suppressive dose. In addition, he received calcium D and Rocaltrol (calcitriol).
The post-ablation whole-body scan with 131I depicted no remnant functioning thyroid tissue and no clear indication of distance metastasis was noted throughout the body
After the mentioned period, the patient was referred to us with complaints related to hypercalcaemia (Severe Nausea and vomiting, Constipation, Lethargy and fatigue) as a probability of drug overdose, (Laboratory test were reported as Ca: 11.5 mg/dl and P: 2.4 mg/dl). Moreover, he expressed a sudden weight loss over the recent 6 months. Owing to a paucity of response to cessation of all drugs, we decided to initiate pamindronate which accompanied with appropriate response.
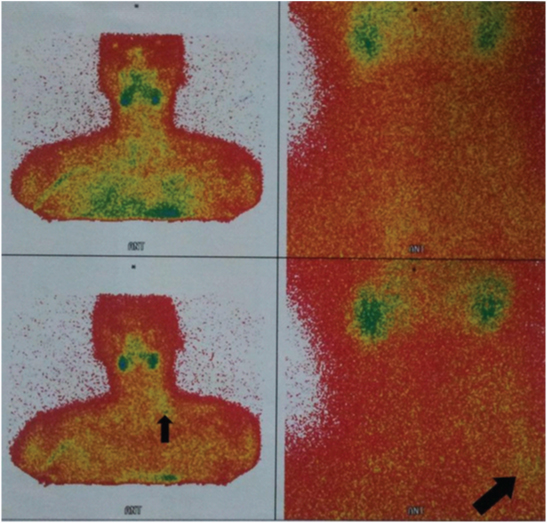
Three weeks later, all the symptoms were repeated. In our experience, this patient arrives with the clinical suspicion of Parathyroid Disease. In addition, further investigation of biochemical analysis showed raised serum calcium and parathyroid hormone (PTH) levels (1311 pg/mL) that confirmed hyperparathyroidism. On presentation, his calcium level was 13 mmol/L, P level 2 pmol/L, and creatinine level of 1 mg/dl. He was screened for multiple endocrine neoplasia which turned out to be negative. Despite the neck imaging was performed by sestamibi scintigraphy [Table/Fig-2]; ultrasonography recognized one firm nodule at the site of the right parathyroid.
A 99m Tc MIBI isotope scans of parathyroid in a 21-year-old
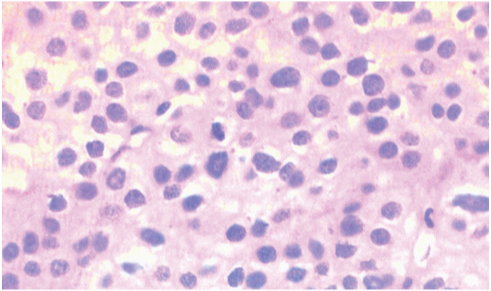
Thus, the patient underwent a total Para-thyroidectomy. Histopathological examination confirmed parathyroid carcinoma [Table/Fig-3]. After the operation, the patient developed persistent Hypercalcaemia. Residual tumour was suspected, as his postoperative PTH level remained elevated. Although whole-body sestamibi scan did not confirm this. However, all the therapeutic interventions were done, his hypercalcaemia worsened. His hypercalcaemia was difficult to manage despite using intravenous hydration and intravenous calcitonin. No significant improvement was shown in serum calcium levels. Subsequently, the patient’s disease progressed and his PTH level was aroused. He became symptomatic, with nausea, anorexia, weight loss, muscle weakness and changes in sensorium. The patient died 3 weeks after his presentation in one of the resistant calcium crisis.
Parathyroid carcinoma: high mitotic activity in the center of photo is visible
Discussion
Primary hyperparathyroidism (HPT) is the most common cause of hypercalcaemia. In less than 1% of cases, this condition results from parathyroid carcinoma [1,2]. Hürthle cell carcinoma accounts for about 5% of differentiated thyroid carcinoma. Separate occurrence of thyroid and parathyroid carcinoma in patients is extremely rare, and according to published reports, only 7 patients with concurrent parathyroid and papillary thyroid carcinomas have been documented [3–7]. Since the separate occurrence of both disease processes can complicate patient management via untreated hypercalcaemia, unrecognized thyroid cancer, and the requirement of re-operative neck surgery, patients should be screened for both disease entities carefully [8,9]. Here we report a case with the unusual existence of both non-medullary thyroid (Hürthle cell carcinoma), and parathyroid carcinoma. This constellation has not been reported previously.
In this case, the patient presented with thyroid cancer manifestation. He became hypocalcaemic after thyroid surgery which is a routine complication but in continuation became hypercalcaemic even after withdrawal from calcium and vitamin D supplementation. Further, he was diagnosed with parathyroid carcinoma. Even though parathyroid carcinoma in not a prevalent aetiology of parathyroid adenoma, the number of such cases become greater in parallel to the increase in hyperparathyroidism prevalence. Several researches showed that accompaniment of thyroid and parathyroid cancer is incidental. However, other studies point to increase in insulin-like growth factors known as goitrogenic factor to be the most responsible cause for this event [2,10,11].
Only 2% of patients with parathyroid carcinoma had non-functioning tumours. These patients had decreased life expectancy, most likely because of delay in presentation (absence of typical symptoms of hypercalcaemia) and therefore present with aggressive tumour biology [2].
[Table/Fig-4] shows that 5 out of 6 patients have functioning Parathyroid carcinoma (including our patient), and their PTH levels was elevated as high as 15 to 100 fold above normal reference values. This finding suggests that it might be useful to check both calcium and PTH levels in every patient that presents with thyroid cancer and is candidate for thyroid surgery in order to prevent additional surgery and considering the possibility of hidden incidental parathyroid carcinoma.
Clinical features of 6 patients with co (co removed) existence of parathyroid and thyroid carcinoma
| Reference | Sex | Age | Calcium (mg/dL) | PTH (pg/mL) | Parathyroid Size (cm) | Carcinoma Location | Thyroid Carcinoma | Associated Parathyroid Disease | Surgical Treatment | Outcome |
|---|
| Kurita et al.,1979 | F | 68 | 12.2 | 6,300 | 4.2×3.2×2.4 | Left | Papillary | None | En bloc resectionUnknown | Postop normocalcaemiaDied from metastaticparathyroid carcinoma |
| Christmaset al., 1988 | F | 62 | Hypercalcaemia | Unknown | Unknown | LowerUnknown | Follicular | None | Total thyroidectomyParathyroidectomy(Excision of 2hyperplastic glands) | Normocalcaemia(1 year) |
| Savli et al.,2001 | F | 47 | Normal | N.D. | Unknown | Unknown | Papillary | Hyperplasia | En bloc resection | Normocaclemia(2 years) |
| Schoretsanitiset al., 2002 [9] | F | 55 | 14.2 | >1,000 | 3×3 | LeftLower | Papillary(Follicularvariant) | None | Total thyroidectomyLeft Parathyroidectomy | Normocalcaemia(6 years) |
| Lin et al.,2005 [8] | M | 38 | 16.5 | 351 | 4×3×3 | LeftLower | Papillary | Two enlargedparathyroidglands oncontralateralside (?) | En bloc resection | Persistent hypercalcaemiaafter resection of parathyroidcarcinoma. |
| Goldfarbet al., 2009 [6] | M | 58 | 14.4 | 2,023 | 3.4×3.3×2.2 | LeftLower | Papillary | Contralateralparathyroidadenoma | | Normocalcaemia afterexcision of contralateralparathyroid adenoma (1 year) |
| PresentCase | M | 21 | 11.5 | 1311 | Unknown | Unknown | Hürthle | ParathyroidCarcinoma | Left thyroid lobectomy,ischmectomy and cervicallymph node dissection | Persistent hypercalcaemiaafter left lobectomy of thyroid,and finally expire |
The presence of parathyroid carcinoma in any young individual like our patient should bring to mind familial syndromes such as HPT-JT syndrome. In this syndrome we expected positive family history, other masses and other presentations such as Jaw osteoma or renal disorders (e.g. kidney cysts or renal hematomas, etc..). None of the aforementioned symptoms were present in our patient according to examinations (and paraclinic data from beginning of disease). Although absence of these symptoms would not rule out the possibility of this syndrome, but it diminishes the chance of having it. The definite diagnosis can be reached by assessing gene mutations of HRPT2 gene. However, due to sanctions and lack of facilities, we didn’t have means to evaluate these mutations inside our country.
Rapid mitotic activity or fibrosis, are not limited to parathyroid carcinoma and could also be seen as reactive changes of the first surgery. In the second surgery in which the parathyroid cancer diagnostic was set, we observed invasion of blood vessels in tissue evaluations following the second surgery as well as pathological changes in favour of parathyroid carcinoma. These findings are strongly in favour of malignancy and rule out reactive changes after the first surgery.
These explain the importance of pre and intraoperative evaluation to detect incidental thyroid and parathyroid pathology before performing a parathyroidectomy for hyperparathyroidism or a thyroidectomy for a thyroid carcinoma to avoid another surgery and delay in diagnosis at extremely stages after disease progression.
Pathologic changes called oncocytic changes are not exclusive to Hürthle Cell and could involve other endocrine tissues such as parathyroid. However, the pathologic features of the first tumour which we differentiated as Hürthle Cell possessed other Hürthle Cell typical characteristics. These included cellular enlargement, polygonal shape, well-defined borders, hyperchromatic nuclei and macronucleus cells called “cherry pink”, in addition to eosinophilic cytoplasm. Moreover, if the tumours in the thyroid had primary parathyroid origin, we would expect the patient to show clinical signs in favour of hypercalcaemia or elevated serum calcium in examinations before the first surgery for thyroid. However, patient did not show signs of hypercalcaemia until long after surgery. Development of hypocalcaemia in the patient after the first thyroid surgery is an expected and normal side effect after any thyroid surgery and we cannot necessarily attribute patient’s calcium drop after surgery to the resection of malignant parathyroid tissue.
Conclusion
Although concurrent parathyroid adenoma and thyroid carcinoma is rare, they can and do coexist. Preoperative assessment such as serum calcium and PTH levels, may be useful for detecting occult primary hyperparathyroidism. Suitable treatment for the parathyroid disease including surgical resection should be performed for the prevention of the adverse disease resulting from the untreated parathyroid disease after thyroid resection. Therefore, when a patient with thyroid cancer is diagnosed, checking for serum calcium is advised. This is considered a useful method for detecting possible incidental parathyroid lesion and screening the probable concealed parathyroid pathology.
[1]. Mahmoodzadeh H, Harirchi I, Hassan Esfehani M, Alibakhshi A, Papillary thyroid carcinoma associated with parathyroid adenomaActa medica Iranica 2012 50(5):353-54. [Google Scholar]
[2]. Javadi H, Jallalat S, Farrokhi S, Semnani S, Mogharrabi M, Riazi A, Concurrent papillary thyroid cancer and parathyroid adenoma as a rare condition: a case reportNuclear medicine review Central & Eastern Europe 2012 15(2):153-55. [Google Scholar]
[3]. Chaychi L, Belbruno K, Golding A, Memoli V, Unusual manifestation of parathyroid carcinoma in the setting of papillary thyroid cancerEndocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2010 16(4):664-68. [Google Scholar]
[4]. Marcy PY, Thariat J, Sudaka A, Poissonnet G, Synchronous parathyroid and papillary thyroid carcinomasThyroid : official journal of the American Thyroid Association 2009 19(10):1131-33. [Google Scholar]
[5]. Baumann K, Weichert J, Krokowski M, Diedrich K, Banz-Jansen C, Coexistent parathyroid adenoma and thyroid papillary carcinoma in pregnancyArchives of gynecology and obstetrics 2011 284(1):91-94. [Google Scholar]
[6]. Goldfarb M, O’Neal P, Shih JL, Hartzband P, Connolly J, Hasselgren PO, Synchronous parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma in a patient with severe and long-standing hyperparathyroidismEndocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2009 15(5):463-68. [Google Scholar]
[7]. Chang MC, Tsai SC, Lin WY, Dual-phase 99mTc-MIBI parathyroid imaging reveals synchronous parathyroid adenoma and papillary thyroid carcinoma: a case reportThe Kaohsiung journal of medical sciences 2008 24(10):542-47. [Google Scholar]
[8]. Lin SD, Tu ST, Hsu SR, Chang JH, Yang KT, Yang LH, Synchronous parathyroid and papillary thyroid carcinomaJournal of the Chinese Medical Association : JCMA 2005 68(2):87-91. [Google Scholar]
[9]. Schoretsanitis G, Melissas J, Kafousi M, Karkavitsas N, Tsiftsis DD, Synchronous parathyroid and papillary thyroid carcinoma: a case reportAmerican journal of otolaryngology 2002 23(6):382-85. [Google Scholar]
[10]. Cheung L, Howlett D, El Teraifi H, Kirkland P, Association of synchronous medullary and papillary thyroid carcinomas with primary hyperparathyroidism: first case report and literature reviewThe Journal of laryngology and otology 2014 128(6):565-68. [Google Scholar]
[11]. Colin IM, Denef JF, Lengele B, Many MC, Gerard AC, Recent insights into the cell biology of thyroid angiofollicular unitsEndocrine reviews 2013 34(2):209-38. [Google Scholar]