Introduction
Diarrhea is one of the most common diseases in infants and young children in developed and developing countries. In Walker et al., study, the prevalence of diarrhea decreased in 2010 compared to 1990, with the highest incidence among infants 6-11 months [1]. In contrast to adults, in whom the most common etiologic agent is Clostridium difficile [2], nosocomial diarrhea in children is usually due to viruses circulating in the community such as rotavirus, enteric adenovirus, astrovirus, norovirus [3], and torovirus [4].
Adenoviruses are double-stranded, non enveloped DNA viruses. There are at least 51 distinct human adenovirus serotypes. These types classified into six species, A to F, cause human infection. Some adenovirus serotypes are associated primarily with respiratory tract diseases, and others are associated primarily with gastroenteritis (types 40, 41 and to a less extent, 31). Infection in infants and children can occur at any age. Health care-associated transmission of adenoviral respiratory tract and gastrointestinal tract infections may occur in hospital [5].
Enteric strains of adenoviruses are transmitted by the fecal-oral route. Adenoviruses do not demonstrate the marked seasonality of other respiratory tract viruses. Enteric diseases occur throughout the year and primarily affect children younger than four years [5].
Almost 50% of children under five years have been seropositive for antibody of adenovirus type 40 (Ad40) and adenovirus type 41 (Ad41) in Asia, Europe, and South America [6].
Ad40 and Ad41 have recently been recognized as important etiologic agents of gastroenteritis in children [7]. Herrmann et al., have reported that Ad40 and Ad41 are associated with a small proportion (2.0%) of gastroenteritis in Thai children attending an outpatient clinic in Bangkok, which is very similar to that reported in Rio de Janeiro, Brazil (1.9%) [8,9].
In study of Modarres et al., enteric adenovirus infection in infants and young children with acute gastroenteritis in Tehran was assessed and Ad40 and Ad41 were detected in 27 (2.6%) samples [10], but few studies have been yet conducted in Iran to investigate the prevalence of nosocomial diarrhea due to Ad40 and Ad41 in children, so this study is done to determine the prevalence of Ad40 and Ad41 nosocomial diarrhea among children less than five years at a paediatric center in Shahrekord, southwest Iran.
Materials and Methods
For this prevalence study conducted from December, 2010 to December, 2011, the protocol was approved by the ethic committee of the University. Informed consent was obtained from the participants’ parents. The study population consisted of all patients of 6-60 months, who were admitted to the paediatric ward of the hospital because of diseases other than diarrhea. Fecal specimens were obtained from 100 children with signs of nosocomial diarrhea admitted to the hospitals and health centers in Shahrekord. The same pediatrician visited the patients every day. Fecal specimens were collected from any children and investigated. Nosocomial diarrhea was defined as occurring more than 72 hours after admission to hospital for reasons other than diarrhea. The fecal samples were transferred daily to the Cellular and Molecular Research Center, Shahrekord University of Medical Sciences in refrigerated boxes. Each specimen was stored at -70°C for later use. The methods used in this study are very similar to another study [11].
The stool specimens were obtained from 100 diarrhea patients less than five years admitted to Hajar Hospital of Shahrekord. The samples were transferred to the laboratory in sterile plastic falcon tubes (15 ml) under refrigerated conditions and stored at -20°C until analysis. The DNA virus was extracted from 200 mg stool specimens using a DNA extraction kit (DNPTM Kit Cinna Gen, Iran) according to the manufacturer’s procedure. The yield of DNA was quantified after electrophoresis in 1% agarose gel containing 0.5 μg/ml of ethidium bromide.
The primers of polymerase chain reaction (PCR) for detection of ad40 were ad40F: 5’-GCCCGTGCCACCGATACCTAC -3’ and ad40R: 5’-ACTTTGTAAGAGTAGGCGGTTTCC -3’.
The ad40 F primer was designed in this research and the sequence of ad40 R primer was obtained from Jiang et al., study [12]. The size of amplicon was 152 bp. The ad40R primers sequence obtained from Xu et al., study, ad41F: 5’-ACTTAATGCTGACACGGGCAC -3’ and ad41R: 5’-TAATGTTTGTGTTACTCCGCTC -3’ [13], were used for amplification of Ad41 and the size of amplicon was 541bp.
PCR was carried out in 25 μl total reaction volumes, each containing 2.5 μl of 10X PCR buffer, 1.5 mM MgCl2, 100 ng of template DNA, 0.2 pM of each primer, 0.2 μl dNTPs, and 1 unit of Taq DNA polymerase (Fermentas, Germany).
The amplification reaction consisted of 5 min of predenaturing at 95°C, followed by 33 cycles of 1 min denaturation at 94°C, 1 min annealing at 60°C and 65°C for ad40F/R and ad41F/R respectively, and 1 min extension 72°C, and final extension at 72°C for 5 min. The samples were amplified in a Gradient Palm Cycler (Corbett Research, Australia).
The PCR amplification products (15 μl) were separated by electrophoresis in 1.5% agarose gel at 100 V for 30 min in Tris-borate-EDTA buffer, visualized by ethidium bromide staining, illuminated by UV transilluminator and images were obtained in UVIdoc gel documentation systems (UK). A 100 bp DNA ladder (Fermentas) was used as a size reference for PCR assay [12,13].
Statistical Analysis
Analysis of data and investigation of Ad40 and Ad41 were performed by chi-square test in the SPSS version 16 (SPSS, Chicago, IL).
Results
Analysis of PCR products for the presence of Ad40 and Ad41 DNA on 1.5% agarose gel revealed 152 bp for Ad40 and 541 bp for Ad41 [Table/Fig-1].
Gel electrophoresis of adenovirus 40 and adenovirus 41. Lane 1 shows fermentas 100 bp molecular marker. Lanes 2 and 6 are negative controls of adenovirus 41 and adenovirus 40, respectively. Lanes 3 and 4 are positive samples for adenovirus 41 (541 bp) and lanes 7 and 8 are positive samples for adenovirus 40 (152 bp). Lanes 5 and 9 are negative samples for adenovirus 41 and adenovirus 40, respectively.
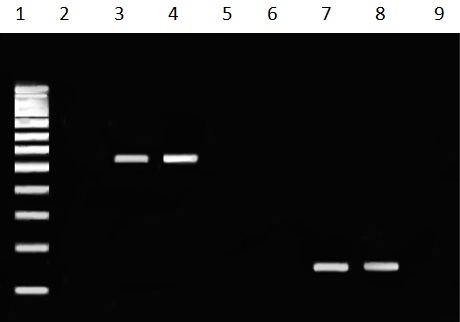
The prevalence of Ad40 and Ad41 in stool specimens among children less than five years with nosocomial diarrhea was 22%. Ad40 and Ad41 DNA was found to be positive in 14/100 (14%), and 8/100 (8%) of diarrheic patients less than five years, respectively.
The mean age of patients was 11.8±15.3 months (range: 6-60 months), and 47% were female. The mean weight of patients was 9±2.7 kg. Chi-square and t-test showed no significant difference among positive samples in sex and age (p>0.05). In addition, there was no relationship between the occurrence of nosocomial diarrhea due to adenoviruses (Ad40 and Ad41) and seasons (p>0.05).
Discussion
Next to rotavirus and norovirus, adenovirus is the most commonly identified viral agent in stools of infants and young children with gastroenteritis [14]. In some studies Ad40 and Ad41 have been detected in 5-15% of patients with diarrhea, and rate of detection was dependent on the level of economic status or geographical region of the study [15,16]; however, in the present study nosocomial adenovirus infections were detected in 22 cases (22%). This is higher than positive samples in study of Kotloff et al., In study of Kotloff et al., Ad40 and Ad41 were responsible for 6.2% of nosocomial diarrhea by a monoclonal antibody-based enzyme-linked immunosorbent assay [17]. This difference is probably related to different laboratory methods in two studies.
In study of Carraturo et al., nosocomial adenovirus infections were detected in seven patients (41.2%, ranging from 38.5% in 2005-2006 to 50.0% in 2006-2007). Overall, 71.4% of cases were children under 24 months [18], but in the present study there was no significant difference among positive samples in sex and age. Dey et al., found that among 101 samples Ad41 was more prevalent (52.5%) followed by Ad40 (24.7%), and total prevalence rate of Ad40 and Ad41 was 50.5% by ELISA and 77.2% by PCR [19]. Molecular epidemiological studies are important in the clinical adenovirus investigation [20].
Adenovirus in clinical specimens can be detected by PCR, enzyme immunoassay, direct fluorescent assay, and virus isolation from cell culture, but PCR was found as a fast, sensitive, and reliable method for the detection of adenoviruses in diarrheal disease; however, it requires special laboratory equipment [21]. In the present study, we analyzed stool samples by PCR. The actual prevalence would have been slightly higher if PCR instead of ELISA had been used as the first step in screening, so the methods of detection are not comparable in different studies. In this study, PCR-based sequence analysis of genomic DNA of adenoviruses confirmed that Ad40 is more prevalent (14%), followed by Ad41 (8%). Kotloff et al., found that Ad41 was more prevalent (68%) than Ad40 (32%) [17]. However, studies in Germany, rural Bangladesh, and the Netherlands showed that the frequencies of these two serotypes were almost similar [14,15,22].
Limitations
The present work suffers from some limitations. This study was conducted in only one hospital, but considering the fact that this hospital is the main referral hospital in the province, so the study subjects may be considered to be a good representative sample. In addition, adenoviruses other than Ad40 and Ad41 were not studied and phylogenetic analysis was not performed.
Conclusion
Regional epidemiological data on adenovirus infections may be important to develop strategies for intervention. Our research confirms that continuous monitoring of nosocomial gastroenteritis caused by enteric adenovirus is needed to monitor the potential adverse effects of these pathologies. We demonstrated the prevalence of Ad40 and Ad41 in a small population of Iranian children with nosocomial diarrhea for the first time. The prevalence of Ad40 and Ad41 per month of the year and at various ages suggested that they could be easily transmitted in a small community with poor hygienic conditions.
[1]. Walker CLF, Perin J, Aryee MJ, Boschi-Pinto C, Black RE, Diarrhea incidence in low-and middle-income countries in 1990 and 2010: a systematic reviewBMC Public Health 2012 12(1):220 [Google Scholar]
[2]. Johnson S, Gerding DN, Clostridium difficile-associated diarrheaClin infect Dis 1998 26(5):1027-34. [Google Scholar]
[3]. Kapikian A, Overview of viral gastroenteritisArch Virol Suppl 1996 12:7 [Google Scholar]
[4]. Jamieson FB, Wang EEL, Bain C, Good J, Duckmanton L, Petric M, Human torovirus: a new nosocomial gastrointestinal pathogenJ Infect Dis 1998 178(5):1263-69. [Google Scholar]
[5]. Adenovirus Infections. Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. [5]2009 Red Book: Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Paediatrics; 2009:204–6 [Google Scholar]
[6]. Singh-Naz N, Rodriguez W, Kidd A, Brandt C, Monoclonal antibody enzyme-linked immunosorbent assay for specific identification and typing of subgroup F adenovirusesJ Clin Microbiol 1988 26(2):297-300. [Google Scholar]
[7]. Uhnoo I, Wadell G, Svensson L, Johansson M, Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young childrenJ Clin Microbiol 1984 20(3):365-72. [Google Scholar]
[8]. Herrmann JE, Blacklow NR, Perron-Henry DM, Clements E, Taylor DN, Echeverria P, Incidence of enteric adenoviruses among children in Thailand and the significance of these viruses in gastroenteritisJ Clin Microbiol 1988 26(9):1783-86. [Google Scholar]
[9]. Leite J, Pereira H, Azeredo R, Schatzmayr H, Adenoviruses in faeces of children with acute gastroenteritis in Rio de Janeiro, BrazilJ Med Virol 1985 15(2):203-9. [Google Scholar]
[10]. Modarres S, Jam-Afzon S, Modarres F, Enteric adenovirus infection in infants and young children with acute gastroenteritis in TehranActa Med Iran 2006 44(5):349-53. [Google Scholar]
[11]. Khoshdel A, Parvin N, Doosti A, Eshraghi A, Prevalence and molecular characterization of rotaviruses as causes of nosocomial diarrhea in childrenTurk J Paediatr 2014 56(5):469-74. [Google Scholar]
[12]. Jiang S, Dezfulian H, Chu W, Real-time quantitative PCR for enteric adenovirus serotype 40 in environmental watersCan J Microbiol 2005 51(5):393-98. [Google Scholar]
[13]. Xu WH, McDonough MC, Erdman DD, Species-specific identification of human adenoviruses by a multiplex PCR assayJ Clin Microbiol 2000 38(11):4114-20. [Google Scholar]
[14]. Oh DY, Gaedicke G, Schreier E, Viral agents of acute gastroenteritis in German children: prevalence and molecular diversityJ Med Virol 2003 71:82-93. [Google Scholar]
[15]. Jarecki-Khan K, Tzipori S, Unicomb L, Enteric adenovirus infection among infants with diarrhea in rural BangladeshJ Clin Microbiol 1993 31(3):484-89. [Google Scholar]
[16]. Blacklow NR, Greenberg HB, Viral gastroenteritisNew England J Med 1991 325(4):252-64. [Google Scholar]
[17]. Kotloff KL, Losonsky GA, Morris JG, Wasserman SS, Singh-Naz N, Levine MM, Enteric adenovirus infection and childhood diarrhea: an epidemiologic study in three clinical settingsPaediatrics 1989 84(2):219-25. [Google Scholar]
[18]. Carraturo A, Catalani V, Tega L, Microbiological and epidemiological aspects of rotavirus and enteric adenovirus infections in hospitalized children in ItalyNew Microbiol 2008 31(3):329-36. [Google Scholar]
[19]. Dey RS, Ghosh S, Chawla-Sarkar M, Panchalingam S, Nataro JP, Sur D, Circulation of a novel pattern of infections by enteric adenovirus serotype 41 among children below 5 years of age in Kolkata, IndiaJ Clin Microbiol 2011 49(2):500-05. [Google Scholar]
[20]. Okitsu-Negishi S, Nguyen TA, Phan TG, Ushijima H, Molecular epidemiology of viral gastroenteritis in AsiaPaediatr Int 2004 46(2):245-52. [Google Scholar]
[21]. Allard A, Girones R, Juto P, Wadell G, Polymerase chain reaction for detection of adenoviruses in stool samplesJ Clin Microbiol 1990 28(12):2659-67. [Google Scholar]
[22]. de Jong J, Kapsenberg JG, Muzerie C, Wermenbol A, Kidd A, Wadell G, Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stoolJ Med Virol 1983 11(3):215-31. [Google Scholar]