Case Report
A 54-year-old Caucasian male presented with a 12-month history of relatively slow growth of a generalized mass in the keratinized gingiva of both jaws. The patient was referred to the Department of Periodontology for consultation after a potential diagnosis of periodontal disease. The patient had a history of kidney transplant that had been treated with cyclosporine (500 mg/day) for the previous four years. Furthermore, the patient has been using nifedipine (30 mg/day) for the treatment of hypertension, and warfarin (5 mg/day) for the prevention of prosthetic heart valve thromboembolism since three years.
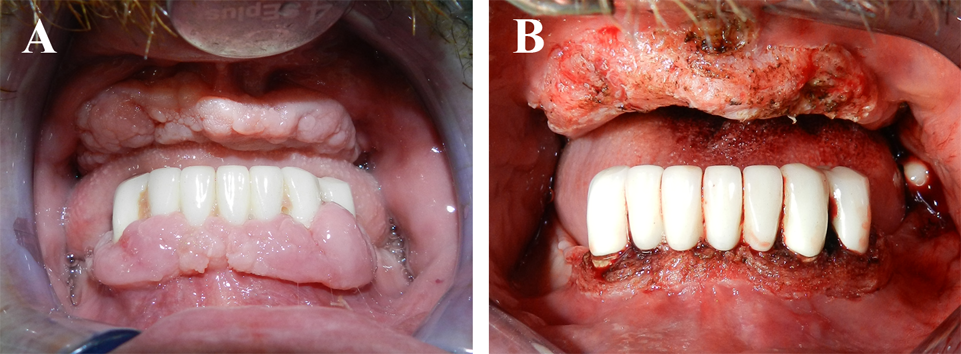
Physical and extraoral examination of the patient were normal. The gingival lesions extended from the mucogingival line around the mandibular teeth and edentulous maxillary ridge were mulberry shaped, firm, pale red, and resilient, a lobulated surface with no tendency to spontaneous bleeding and showed no hyperpigmentation or discoloration [Table/Fig-1a,b].
Generalized gingival enlargements are seen as mulberry shaped, firm, pale red, and resilient, with a lobulated surface (a) intraoperative clinical photograph after diode laser-assisted gingivectomy procedure (b)
The gingival enlargements caused formation of periodontal pockets/pseudopockets. The periodontal condition of the teeth associated with gingival overgrowth was determined by Gingival Index (GI), Probing Depth (PD) and mobility scores [Table/Fig-2]. Furthermore, Vertical Gingival Overgrowth Index (GO index) was used for grading of gingival enlargements. In addition, horizontal gingival overgrowth was measured in the buccal-lingual direction in all interdental papilla according to the MB index [1].
Periodontal characteristics of the teeth associated with gingival hyperplasia at baseline
| Tooth #43 | Tooth #33 | Tooth #34 |
|---|
| Facial | Lingual | Facial | Lingual | Facial | Lingual |
|---|
| GI index | 2.2.2 | 2.2.2 | 2.2.2 | 2.2.2 | 2.2.2 | 2.2.2 |
| PD (mm) | 4.4.6 | 4.5.6 | 6.5.7 | 4.4.6 | 6.4.4 | 4.3.4 |
| GO index | 2.2.3 | 2.2.2 | 2.2.3 | 2.2.2 | 3.3.2 | 2.2.2 |
| MB index | 1 | 1 | 2 | 2 | 2 | 2 |
| Mobility | 0 | 1 | 0 |
Periodontal condition of the teeth was determined by Gingival Index (GI), Probing Depth (PD), Vertical Gingival Overgrowth Index (GO index), and horizontal gingival overgrowth index in the interdental area (MB index). A periodontal probe was used to measure 6 sites per tooth: 1) mesio-buccal, 2) mid-buccal, 3) disto-buccal, 4) mesio-lingual, 5) mid-lingual, and 6) disto-lingual. Two scores were obtained for MB index, one for the buccal papilla and another for the lingual papilla.
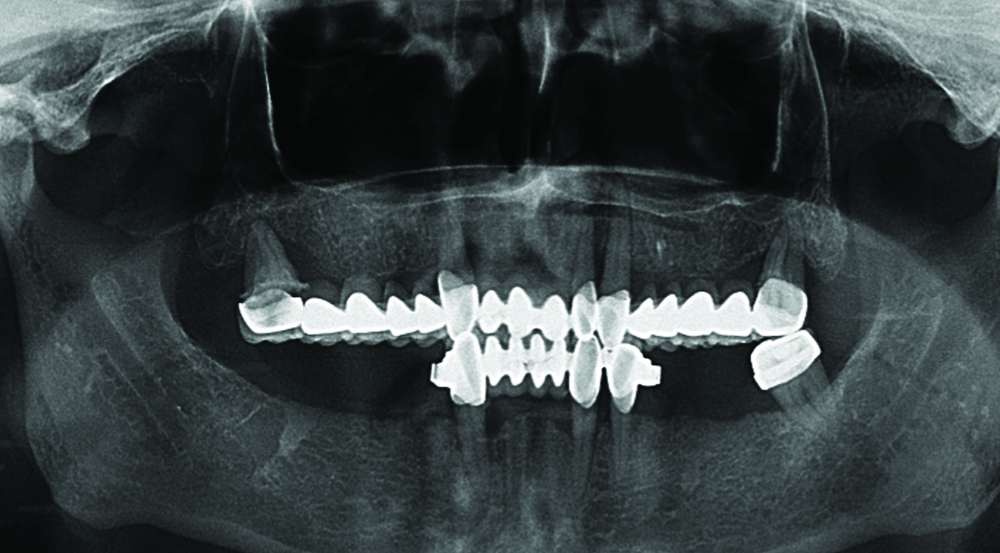
Generalized alveolar bone loss was observed on the panoramic radiograph taken one month before referring to the Department of Periodontology [Table/Fig-3].
Panoramic radiographic image of 54-year-old male patient’s baseline periodontal status. Severe alveolar bone loss is evident around the maxillary teeth
The extractions of the teeth 18, 13, 22, 23, and 28 were done previously by the referring dentist because of advanced periodontal attachment and bone loss.
The patient, who was using an immunosuppressive drug, was recommended for medical consultation because of the increased susceptibility to possible bacteraemia. Furthermore, the drugs were asked to be changed to equivalents that are not associated with risks for gingival enlargement. After medical consultation, cyclosporine was changed to tacrolimus [2–4], and nifedipine was changed to captopril (100 mg/day). The patient’s blood viral tests were negative, and complete blood count and biochemical profile were within normal limits. Moreover, the patient’s medical doctor preferred to discontinue the antiaggregant drug and prescribed antibiotic prophylaxis to reduce the risk of infective endocarditis. The patient was instructed to start taking enoxaparine (0.6 cc twice a day) instead of warfarin five days before the surgery. When the international normalized ratio value was 2.3, dental surgical approaches were applied under antibiotic prophylaxis of amoxicillin (2000 mg single dose) as recommended by the American Heart Association [5]. The patient was directed to restart warfarin therapy one day after the surgery.
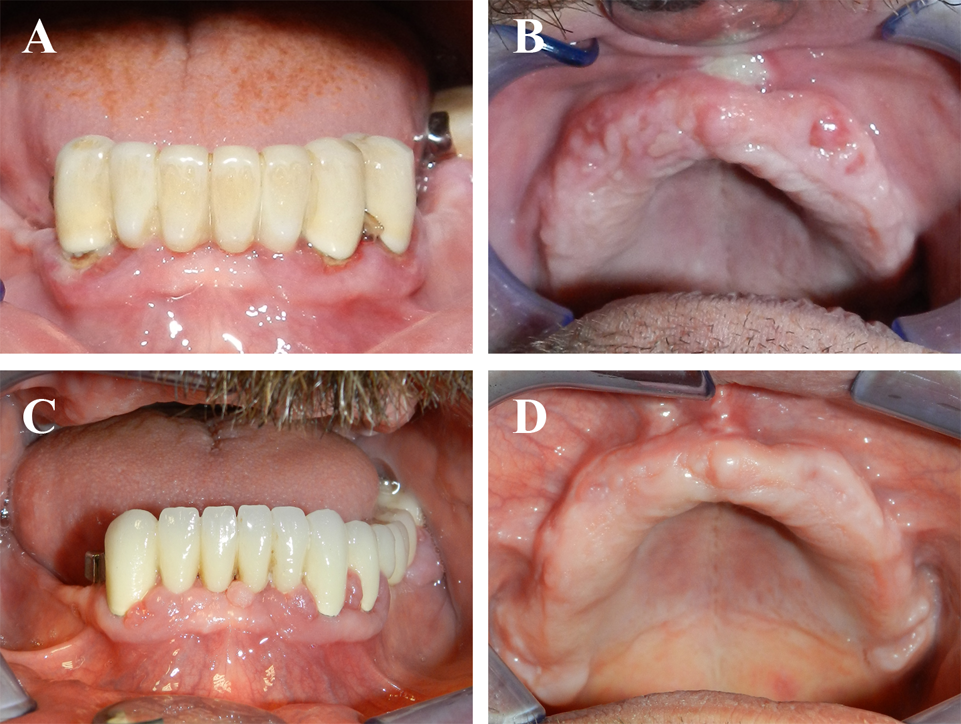
The patient received periodontal therapy and oral hygiene instruction sessions. After two months, the patient underwent surgical therapy consisting of a laser-assisted gingivectomy. After anesthetizing the area, sounding of the underlying alveolar bone was performed with a periodontal probe to determine the thickness of the gingival biotype and amount of tissue to be removed. An excisional biopsy of the gingiva was performed with a #15 surgical blade, the specimen including gingival epithelium and connective tissue was excised and stored immediately in formalin solution for further histopathological examination. Subsequently, the periodontal pockets on each surface were explored with a periodontal probe and marked with a pocket marker. Kirkland periodontal knives were used for incisions on the facial and lingual surfaces. Orban periodontal knives were used for interdental incisions. The external bevel incisions were started apical to the points marking the course of the pockets and were directed coronally to a point between the base of the pocket and the tooth without exposing the alveolar bone. Residual gingival enlargements were removed via diode laser (940 nm), and scaling and root planning were performed [Table/Fig-1]. Maxillary anterior frenectomy was also performed to increase the mechanical strength of the partial prosthesis that was planned to be renewed. Postoperative second-generation cephalosporin antibiotic (Cefaclor 250 mg/day), paracetamol (500 mg/day), and benzydamine hydrochloride rinse (twice a day) were prescribed. Oral hygiene instructions were given to the patient before and after the surgery. The follow-up images of the mandibular teeth [Table/Fig-4a], and the edentulous maxilla [Table/Fig-4b] were illustrated on postoperative days 20. Furthermore, images of the healing process of the mandible [Table/Fig-4c], and the maxilla [Table/Fig-4d] as shown on postoperative days 80.
The healing process around the mandibular teeth (A), and in the maxilla (B) at postoperative days 20. Images of the healing process of the mandible (C), and the maxilla (D) are seen on postoperative days 80
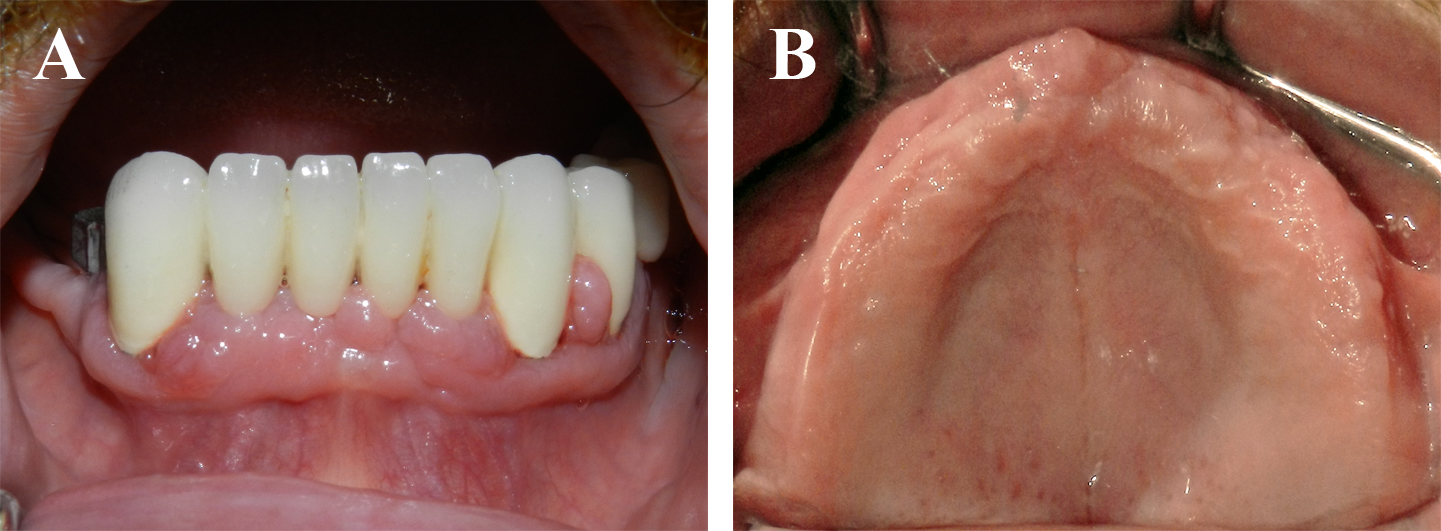
After renewal of the mandibular anterior metal–porcelain restoration, a removable total prosthesis on maxilla and a partial removable prosthesis on mandibular were given to the patient [Table/Fig-5].
The image shows the situation after renewal of the dental prosthesis
At five months postoperative, it was seen that the gingival enlargements around the mandibular teeth relapsed [Table/Fig-6a], however no gingival overgrowth was observed in the edentulous maxilla [Table/Fig-6b], so we decided to repeat the diode laser-assisted surgery.
Images illustrate relapse of gingival enlargements around the mandibular tooth at postoperative five months (A), no sign of gingival enlargement was seen in the maxilla (B)
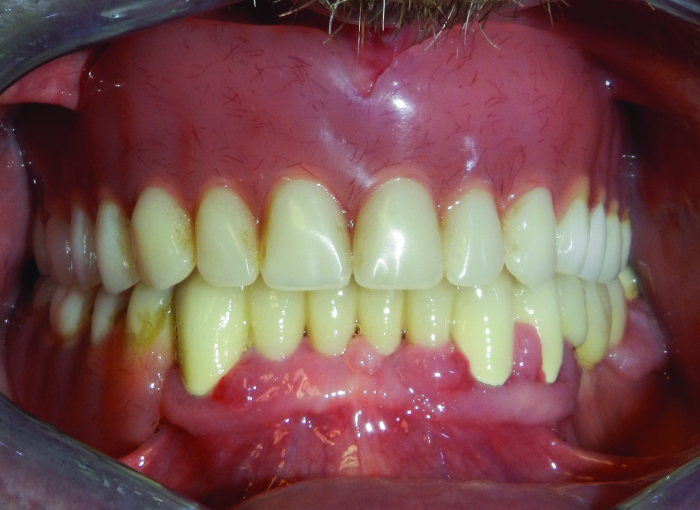
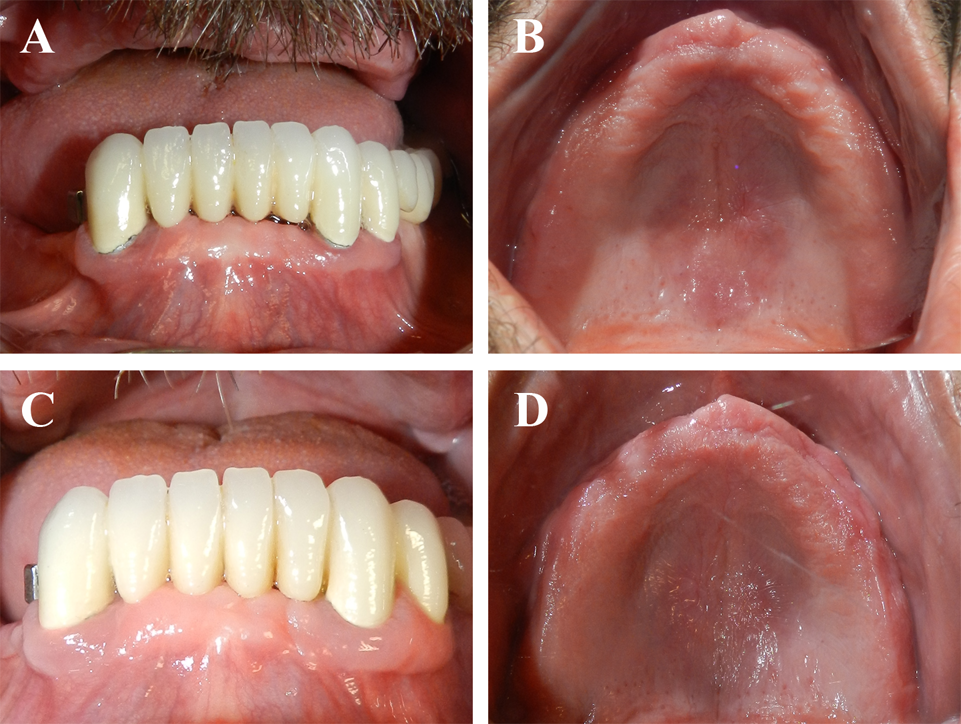
The patient was followed-up postoperatively at 6 months and no relapse was seen around the mandibular teeth [Table/Fig-7a], and in the maxilla [Table/Fig-7b], and at 18 months around the mandibular teeth [Table/Fig-7c], and in the maxilla [Table/Fig-7d].
Postoperative intraoral pictures show the clinical health of periodontal tissues six months in the mandibula (A), and in the maxilla (B), and 18 months in the mandibula (C), and in the maxilla (D) after the second surgery. No clinical signs of gingival enlargement in the gingiva were noted
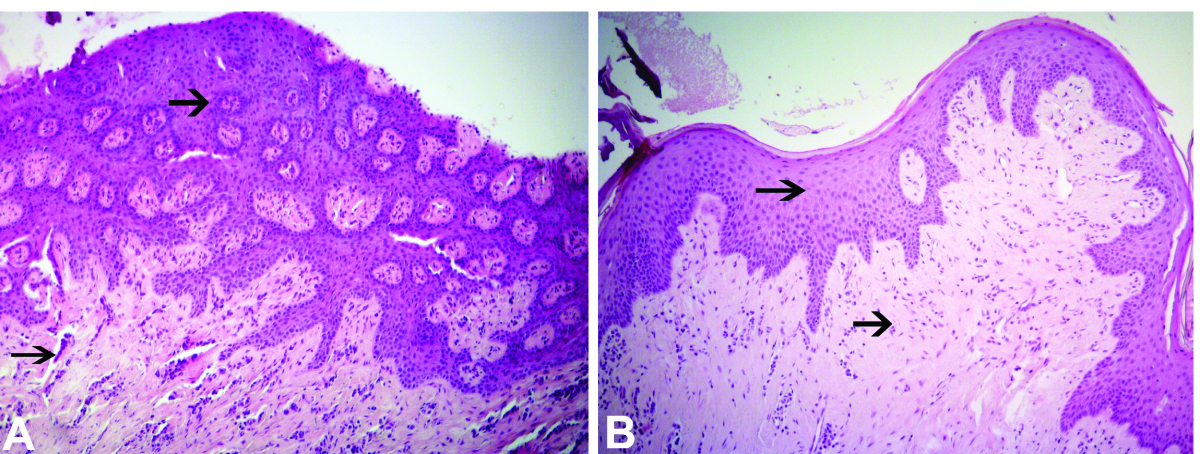
The microscopic findings are presented for the first [Table/Fig-8a], and second surgeries [Table/Fig-8b]. Epithelial proliferation thickness and irregular connective tissue thickness were significantly increased in the first specimen, whereas connective tissue height was normal in the second one.
Proliferated parakeratinized stratified squamous epithelium, dense irregular fibroconnective tissue, and an increased number of blood vessels and fibrotic cells (A). The epithelium stratum was reduced and there was a normal rate of fibrotic cell and connective tissue, in contrast to what was seen in image A (B). Arrows indicate epithelial proliferation and regular connective tissue (H&E, x100).
Discussion
Periodontal diseases modified by medications are becoming more common because of the increased use of these drugs, which are known to induce gingival enlargement. Gingival enlargement has been associated with the administration of immunosuppressant, anticonvulsant, and calcium channel blocker drugs [6].
Cyclosporine A is a potent immunosuppressive agent used systemically to prevent rejection of organ transplants, and to treat several autoimmune diseases, such as lichen planus and pemphigus vulgaris [6]. Gingival overgrowth induced by cyclosporine varies according to different studies from 8% to 70% [6]. The exact mechanism underlying the pathogenesis of cyclosporine-induced gingival enlargement remains unclear [7]. Cyclosporine-induced gingival enlargement initiates as a pebbly enlargement of the interproximal papilla. The enlargement progresses over time, in some cases to cover the entire crown of the tooth. Gingival overgrowth may not only create speech, mastication, tooth eruption, and aesthetic problems, but also increases the susceptibility to periodontitis via the formation of a pathological periodontal pocket/pseudopocket [6].
Hypertension, diabetes, hyperlipidaemia, hirsutism, renal dysfunction, infection, malignancy, neurotoxicity, and gingival hyperplasia are reported to be common side effects of cyclosporine medication [8]. Because organ transplant patients who are administered cyclosporine often have renal hypertension, they are generally prescribed calcium channel blockers such as nifedipine, diltiazem, or verapamil as a vasodilator to control blood pressure [9]. It has been shown that both cyclosporine, a potent immunosuppressive agent, and nifedipine, a calcium channel blocker, independently induce gingival overgrowth [9]. Several studies have revealed marked variations in individual susceptibility to these drugs which suggest a genetic predisposition. A polymorphism in the transforming growth factor beta-1 gene, was represented an independent genetic determinant of severe gingival enlargement in individuals receiving treatment with cyclosporine and calcium channel blockers [10]. Furthermore, cytotoxic T-lymphocyte antigen 4 gene polymorphism was suggested to be a permissive element for cyclosporine-induced gingival enlargement [11].
Most of the indexes for gingival overgrowth evaluation are difficult to reproduce, since they require the preparation of casts, photographs, and slide projection. Therefore, we used both clinically practical vertical (GO index) and horizontal (MB index) indices to quantify gingival overgrowth [1]. The evaluation of gingival enlargement in the area of the interdental papilla that the morphological changes first reveals itself, is the advantage of MB index [1]. Furthermore, together with GO index, they measure both vertical and horizontal components of gingival enlargements, making it easy to detect both initial and advanced gingival enlargements [1].
It is crucial to treat gingival inflammation caused by pathogenic bacterial plaque and increasing the patient’s plaque control because evidence suggests that good oral hygiene and frequent professional removal of plaque decrease the degree of gingival enlargement and improve overall gingival health [1,12]. Afterwards, gingival enlargements should be recontoured to their natural form by conventional surgical treatments, such as gingivectomy and periodontal flap using a scalpel [12], and novel treatment approaches such as diode laser-assisted excision of gingival enlargements. Laser applications in the excision of gingival overgrowth may be a good choice to achieve coagulation effects in such lesions that are rich in blood vessels [13]. Because of the increased gingival vascularization in cyclosporine-induced gingival overgrowth, we preferred diode laser-assisted surgery in this case report. Diode laser application that might alleviate haemorrhagic problems via its haemostatic action was found to be useful in coagulation during surgery and decreased postoperative bleeding.
Treatment choices of drug-induced gingival enlargements that should be decided with the patient’s physician include substituting cyclosporine with another medication. Tacrolimus has similar immunosuppressant effects to those of cyclosporine, and is reported to be superior to cyclosporine with regard to its common adverse effects [2–4]. In this case report, the relapse of the gingival enlargement was noticeable on postoperative day 80 [Table/Fig-4c], and surgical treatment was repeated at postoperative month five [Table/Fig-6a]. Six months after the second postoperation, no relapse was seen [Table/Fig-7].
Alternative treatment options may include azithromycin therapy to induce partial or even complete regression of gingival hyperplasia, especially when administered early in the enlargement process [14,15]. Furthermore, adjunctive use of chlorhexidine has been found to be beneficial in reducing the severity of gingival enlargements [16].
While prescribing postoperative medications after surgery, clinicians should be aware of cyclosporine nephrotoxicity [17], and they should avoid medications that are eliminated primarily through the kidneys. In the present case report, we preferred a second-generation cephalosporin antibiotic, cefaclor, to control any possible postoperative oral infection.
The recurrence of severe gingival overgrowth was observed in 34% of patients medicated with cyclosporine or nifedipine 18 months after periodontal surgery [12]. In another follow-up study, about half of the patients under cyclosporine therapy had a recurrence after gingivectomy [18]. It appears that the risk of recurrence increases as early as 3–6 months following the surgical approach, and positively correlated with poor oral hygiene [12]. Furthermore, attendance at recall appointments, additional dihydropyridine medication, and gingival inflammation were found to be significant risk factors for an increase or recurrence of cyclosporine-induced gingival enlargement [12,18]. Thus, controlling periodontal disease, improving oral hygiene, substituting cyclosporine and dihydropyridine with other medications, complying frequent recall appointments and making a hygienic prosthetic design are important to reduce the risk for recurrence of gingival overgrowth.
In this case report, relapse of gingival enlargements around the mandibular tooth at postoperative five months was observed, however no recurrence was observed in the maxilla. Poor oral hygiene and failure to comply with recall appointments might be the reasons for the recurrence of gingival enlargements. No clinical signs of gingival enlargement in the gingiva were noted 18 months after the second surgery followed by frequent recall appointments and good oral hygiene.
Because of incompatibility between oral soft tissues and prosthesis after treatment of gingival enlargement, renewal of individual dental prosthesis may be needed. To date, no case of diode laser treatment, prosthetic restoration, and follow-up of a cyclosporine and nifedipine combined gingival enlargement has been reported.
Conclusion
In this case report, the patient who was administered cyclosporine and nifedipine had severe gingival enlargements which were treated using a diode laser-assisted surgery. The gingival enlargements were relapsed around the teeth, thus the surgery was repeated. The reasons for the recurrence of gingival enlargements were thought to be poor oral hygiene and incompliance with recall appointments. No relapse was seen after the second postoperation followed by frequent recall appointments and good oral hygiene.
[1]. Miranda J, Brunet L, Roset P, Berini L, Farre M, Mendieta C, Prevalence and risk of gingival enlargement in patients treated with nifedipineJ Periodontol 2001 72(5):605-11. [Google Scholar]
[2]. Hernandez G, Arriba L, Frias MC, Conversion from cyclosporin A to tacrolimus as a non-surgical alternative to reduce gingival enlargement: a preliminary case seriesJ Periodontol 2003 74(12):1816-23. [Google Scholar]
[3]. James JA, Boomer S, Maxwell AP, Reduction in gingival overgrowth associated with conversion from cyclosporin A to tacrolimusJ Clin Periodontol 2000 27(2):144-48. [Google Scholar]
[4]. Hernandez G, Arriba L, Lucas M, de Andres A, Reduction of severe gingival overgrowth in a kidney transplant patient by replacing cyclosporin A with tacrolimusJ Periodontol 2000 71(10):1630-36. [Google Scholar]
[5]. Lam DK, Jan A, Sandor GK, Clokie CM, Prevention of infective endocarditis: revised guidelines from the American Heart Association and the implications for dentistsJ Can Dent Assoc 2008 74(5):449-53. [Google Scholar]
[6]. Kataoka M, Kido J, Shinohara Y, Nagata T, Drug-induced gingival overgrowth–a reviewBiol Pharm Bull 2005 28(10):1817-21. [Google Scholar]
[7]. Hallmon WW, Rossmann JA, The role of drugs in the pathogenesis of gingival overgrowth. A collective review of current conceptsPeriodontol 2000 1999 21:176-96. [Google Scholar]
[8]. Penninga L, Moller CH, Gustafsson F, Steinbruchel DA, Gluud C, Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trialsEur J Clin Pharmacol 2010 66(12):1177-87. [Google Scholar]
[9]. Thomason JM, Seymour RA, Rice N, The prevalence and severity of cyclosporin and nifedipine-induced gingival overgrowthJ Clin Periodontol 1993 20(1):37-40. [Google Scholar]
[10]. Radwan-Oczko M, Boratynska M, Zietek M, Dobosz T, Transforming growth factor-beta1 gene expression and cyclosporine A-induced gingival overgrowth: a pilot studyJ Clin Periodontol 2008 35(5):371-78. [Google Scholar]
[11]. Kusztal M, Radwan-Oczko M, Koscielska-Kasprzak K, Boratynska M, Patrzalek D, Klinger M, Possible association of CTLA-4 gene polymorphism with cyclosporine-induced gingival overgrowth in kidney transplant recipientsTransplant Proc 2007 39(9):2763-65. [Google Scholar]
[12]. Ilgenli T, Atilla G, Baylas H, Effectiveness of periodontal therapy in patients with drug-induced gingival overgrowth. Long-term resultsJ Periodontol 1999 70(9):967-72. [Google Scholar]
[13]. Haytac CM, Ustun Y, Essen E, Ozcelik O, Combined treatment approach of gingivectomy and CO2 laser for cyclosporine-induced gingival overgrowthQuintessence Int 2007 38(1):e54-59. [Google Scholar]
[14]. Nowicki M, Kokot F, Wiecek A, Partial regression of advanced cyclosporin-induced gingival hyperplasia after treatment with azithromycin. A case reportAnn Transplant 1998 3(3):25-27. [Google Scholar]
[15]. Kim JY, Park SH, Cho KS, Mechanism of azithromycin treatment on gingival overgrowthJ Dent Res 2008 87(11):1075-79. [Google Scholar]
[16]. Pilatti GL, Sampaio JE, The influence of chlorhexidine on the severity of cyclosporin A-induced gingival overgrowthJ Periodontol 1997 68(9):900-04. [Google Scholar]
[17]. Xiao Z, Li CW, Shan J, Interventions to improve chronic cyclosporine A nephrotoxicity through inhibiting renal cell apoptosis: a systematic reviewChin Med J (Engl) 2013 126(19):3767-74. [Google Scholar]
[18]. Pernu HE, Pernu LM, Knuuttila ML, Effect of periodontal treatment on gingival overgrowth among cyclosporine A-treated renal transplant recipientsJ Periodontol 1993 64(11):1098-100. [Google Scholar]