Chromoblastomycosis is a form of chronic subcutaneous mycosis, usually occurring in immunocompetent individuals caused by melanised fungi with characteristic visible raised crusted surface lesions and histological hallmark of brown sclerotic bodies. Only a few sporadic cases have been reported from eastern India [1]. It is usually an occupational disease following traumatic implantation of saprophytic fungi, mainly affecting individual in tropical and temperate regions [2]. Diagnosis is often delayed or misdirected due to poor degree of clinical suspicions or confusions with many similar looking conditions. Laboratory confirmations often become difficult due to improper sample collection, inadequate examinations of tissue with scanty fungal bodies or confusion with similar looking artifacts and fungal contaminants. In such a situation, thorough investigation by expert clinicians and microbiologists can only reflect the exact magnitude of the problem.
Materials and Methods
The study has been conducted for a period of 24 months in the Department of Dermatology and Microbiology of a superspciality teaching hospital cum research institute of eastern India. Annual OPD attendance of dermatology department is around 36,000 patients. Inclusion criteria include chronic subcutaneous infections with raised crusty lesions. Out of all these to conclude as chromoblastomycosis either by demonstration of brown sclerotic bodies in tissue [Table/Fig-1] and/or growth of causal dematiaceous fungus from culture in more than one inoculated tubes in first attempt or in repeat test. Other type of lesions as appeared clinically has not been included in present study. History of the suspected patients were taken relevant to their present conditions, e.g. occupation, residence, history of trauma, duration of the lesions, nature of progression, regression if any, associated clinical symptoms like itching, discharge of any fluid, grains etc; pain, liberation of any bad odour etc. Queries regarding associated conditions especially diabetes mellitus or other endocrinopathy, any previous similar lesions etc., history of antimicrobial or other drug therapy were also made accordingly. General examination and examination of the lesions were done in the dermatology OPD. Lymph nodes of the draining areas were examined. Dry crusty materials from the surface of the suspicious lesions were obtained for laboratory investigations by vigorous scrapping and /or by wedge biopsy after having informed consent from them. Sometimes more than one sample was collected in different episodes from the same patient or lesion, to confirm the diagnosis of highly suspected cases. Biopsy materials were collected punch biopsy for flat lesions and wedge biopsy for warty lesions. Tissues were preserved and examined for histo-pathological examination (HPE).
KOH mount showing brown sclerotic bodies (X 40)
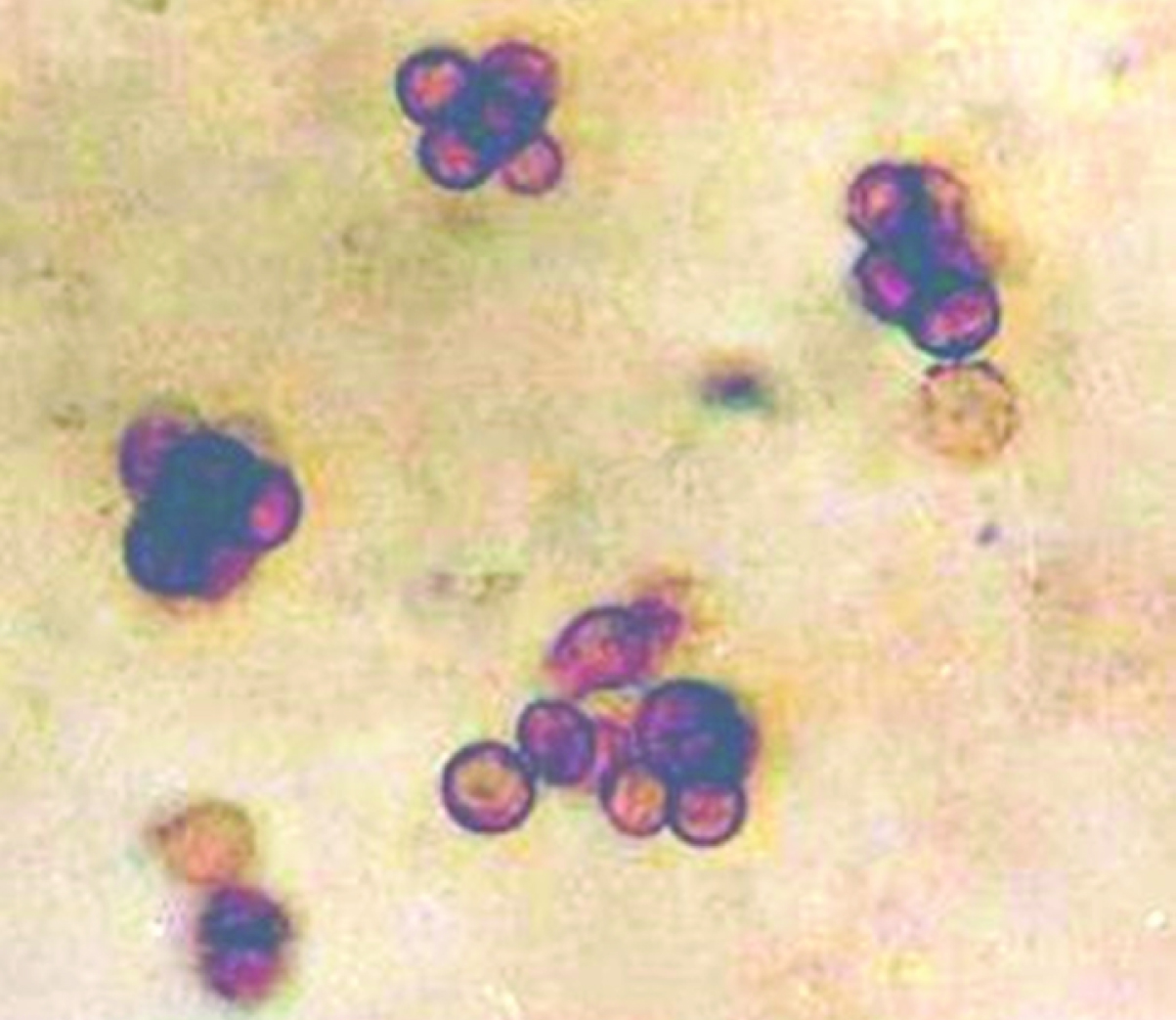
The skin scrapings from the lesions after careful collection were placed over a clean glass slide with few drops of 10% potassium hydroxide and then covered with a clean cover slip. While collecting samples care was taken so as to include materials from depth of the lesions. The assembly was now heated gently over the flame and examined under microscope first in the 10X and then 40X objective lens. Another part of the specimen was inoculated onto several slants of the Sabouraud’s dextrose agar with cycloheximide and/ or chloramphenicol. Tubes were incubated at room temperature (around 250C) in aerobic incubator for 2-4 weeks with periodic inspection for evidence of fungal growth. Once the growth appeared in the slant, the fungal colonies were observed from both obverse and the reverse side. Identification was done based on finding of colony morphology of fungi, KOH wet mount examination, Lactophenol cotton blue (LPCB) tease mount to see their conidiation which had been finally supported by slide culture study. Biopsy materials were stained with Periodic Acid Schiff stain (PAS), Haematoxylin and Eosin and Grocott’s stain.
The patients under study were investigated for other parameters like: complete haemogram, Erythrocyte Sedimentation Rate (ESR), platelet count, blood glucose estimation, urea, creatinine study. They also underwent serological investigation for syphilis, HIV/AIDS. Chest X-ray, Mantoux test and and Zieha-Neelsen staining of the materials from surface lesions or sputum (wherever applicable).
Result
In this 24 months’ study period, 20 suspected cases fulfilling the exclusion and inclusion criteria were studied. Five cases showed characteristic sclerotic bodies from the sample and three of them were found to be culture positive [Table/Fig-2]. Characteristic hallmark i.e. sclerotic bodies couldn’t be detected in neither the rest 15 cases, nor did they yield any relevant fungal growth on culture. Hence, confirmed cases of chromoblastomycosis are five out of 20 suspected cases (25%) in two years. The local incidence of proven cases of chromoblastomycosis in this study is 0.007 per 100 patients attending dermatology OPD per year.
Summary of the findings of the confirmed chromoblastomycosis cases
| Case No. and Localization | KOH microscopy (attempt) | Z-N staining | Biopsy /HPE finding (attempt) | Culture findings (attempt) | Morpho-diagnosis | Managed with | Response to treatment |
|---|
| 1. Left fore arm | Sclerotic body (1st) | No AFB found | Sclerotic body (1st) | growth of fungus (2nd) | Fonsacaea pedrosoi | Oral Itraconazole | Good response |
| 2. Medial aspect of left ankle* | Sclerotic body(3rd) | No AFB found in all three occasion | Sclerotic body (3rd) | Growth of contaminants (1st&2nd)No growth (3rd) | Not applicable | SSKI + debridement + Itraconazole | Moderate to good improvement |
| 3. Right lower thigh, knee | Sclerotic body (1st) | No AFB found | Sclerotic body (1st) | Growth of fungus (1st) | Fonsacaea pedrosoi | oral Itraconazole +SSKI | Moderate Improvement |
| 4. Front of right lower thigh | Sclerotic body(2nd) | No AFB found | Sclerotic body(2nd) | Fungus colony (1st&2nd) | Cladosporium spp. | Itraconazole+ debridement | Moderate to good response |
| 5. Right upper abdomen | Sclerotic body (1st) | No AFB found | Sclerotic body (1st) | Growth of contaminants (1st) and No growth (2nd) | Not applicable | Itraconazole | Moderate Improvement |
*indicates [Table/Fig-6]
Twelve of rest 15 cases were concluded as various other dermatological conditions viz. Lupus vulgaris (2 cases,10%), Atypical mycobacterial infections (3 cases; 10%), Hypertrophic lichen planus (2, 10%), Hypertrophic Discoid lupus erythematosus (DLE) plaque (2, 10%), Wart (1; 5%); fixed cutaneous sporothricosis (1, 5%), zygomycosis (1, 5%) [Table/Fig-3]. Three cases were lost in follow up.
Summary of the negative cases.
| Final diagnosis † | Calcofluor White staining | ZN staining | HPE with special staining(stain) | Culture result | Special comment |
|---|
| Lupus vulgaris (2) | No fungal element | No AFB detected | No AFB detected | No growth | - |
| Atypical mycobacterial infections (3) | No fungal element | AFB detected | AFB detected | Growth of atypical Mycobacteria in LJ media (3) | MPT 64 Ag test -Negative |
| Hypertrophic lichen planus (2) | No fungal element | No AFB detected | Epidermal hyperplasia with changes limited to the tips of the rete-ridges (H&E) | No growth | - |
| Hypertrophic DLE plaque (2) | No fungal element | No AFB detected | Hyperkeratotic pseudoepitheliomatous hyperplasia of epidermis with interface and deep lymphocytic infiltration of basal keratinocyte (H&E) | No growth | - |
| Wart (1) | No fungal element | No AFB detected | Papillomatous epidermal hyperplasia with cytoplasmic vacuolization | No growth | - |
| Fixed cutaeneus sporothricosis (1) | No fungal element | No AFB detected | Nothing significant | Growth of fungus | S. shenkii identified |
| Zygomycosis (1) | Aseptate fungal hyphae seen | No AFB detected | Aseptate fungal hyphae (PAS) | Growth of fungus | Mucor racemosus identified |
† - figures in the parentheses indicate number of cases
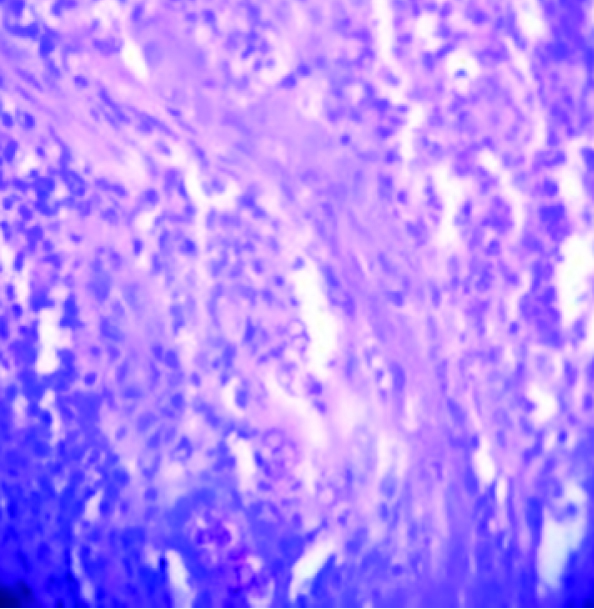
Photomicrograph showing sclerotic bodies in upper dermis (H&E X40)
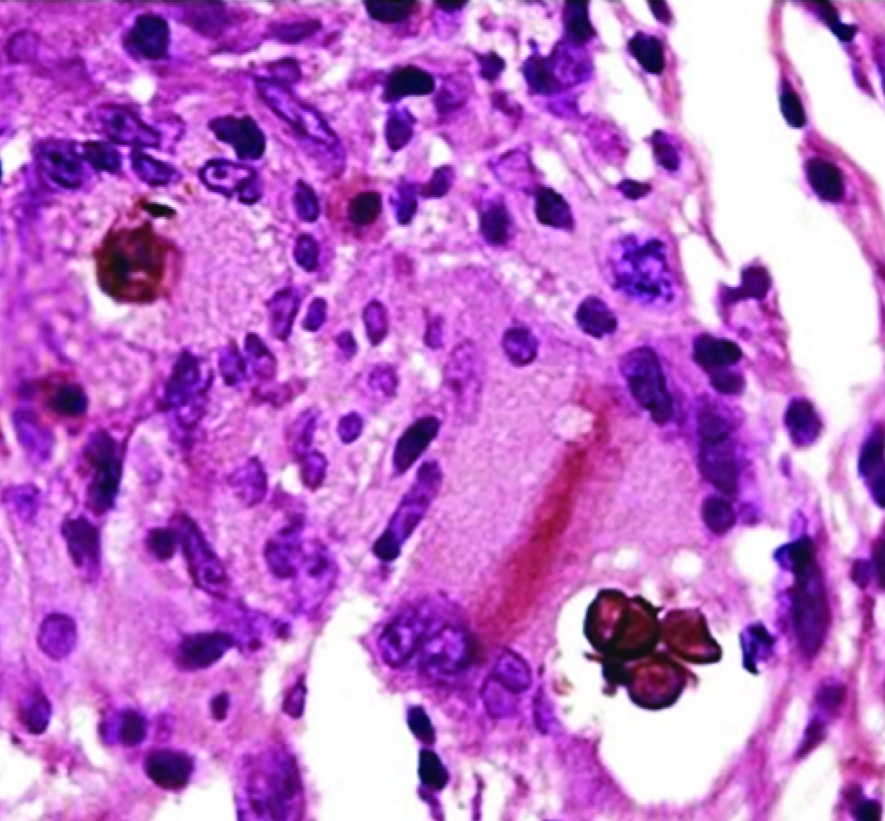
Photomicrograph showing sclerotic bodies in upper dermis (H&E X100)
Discussion
Chromoblastomycosis has been reported infrequently from almost all corners of world but significantly from tropic and subtropical areas. Initial two cases from India were reported by Thomas et al, and, Kakoti and Dey, both in 1957, almost simultaneously [4,5]. Mohapatra et al., have discussed and summarized ten cases of chromoblastomycosis reported from India and Nepal [6]. Aikat reported a case from Chandigarh and Viziam from Vellore, Tamilnadu, India.
Cases have been reported from West Bengal as well, but all were not authentic culture positive or histologically confirmed cases. Chromoblastomycosis usually occurs in agricultural and wood workers. Man to man transmission has not been reported. Typification of chromoblastomycotic lesions was done by Carrion et al., in five different forms as follows -nodules, tumours, plaques, warty lesions and scarring lesions [7]. But the mature lesions show in most cases verrucus or papillomatus appearance that can even amount to elephantiasis in leg or foot if affected. More than one type of lesions may co-exist in same patient. In our study we have found flat cicatricial or plaque lesions. However, verrucous plaque lesions were reported by Bandopadhyay A et al., from West Bengal, India, and Sarina D et al., from South Africa. In the first case culture showed growth of Fonsacaea pedrosoi whereas the second report was based on histopathology with no mention of culture [8,9]. Chromoblastomycosis usually occurs in lower leg, shoulders, arms, hands, buttocks and in abdomen. In present study the lesions involved lower legs, arms, abdomen in the order of frequency [Table/Fig-6]. But De et al., reported a case of chromoblastomycosis in face from Kolkata, India [10]. Another case was reported from Seoul, Korea where lesion occurred in right breast of a female without a history of trauma [11]. Both the reports were silent about isolation of causative fungus. However, chromoblastomycotic keratitis has been reported from Thailand from two patients and both the cases were culture positive to yield Fonsacaea pedrosoi [12].
Lesion of chromoblastomycosis over the medial aspect of left ankle
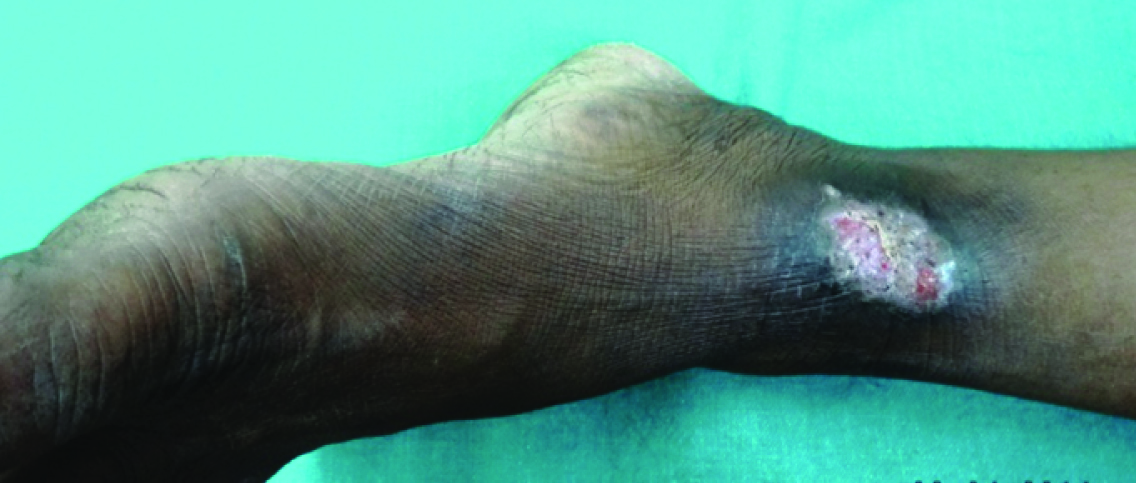
In the present study, fungal growth on culture appeared from three cases out of the five cases which showed sclerotic bodies/ muriform cells [Table/Fig-2]. In one case growth came on second attempt. For the rest two cases growth appeared either on first attempt or on repeated attempts. Fungi were processed/ examined by both KOH wet mount and LPCB tease mount and slide culture as well. Two growths were morphologically identified as Fonsacaea pedrosoi and one as Cladosporium carrionii. Samples from two cases failed to yield any significant fungal growth apart from contaminants. In fungal culture, the colonies may sometimes exhibit more than one type of sporulations due to pleomorphism. Therefore, the final identification is best studied by slide cultures.
A study from Kerala, India, reported a series of 35 cases of chromoblastomycosis with Fonsacaea pedrosoi as commonest isolated pathogen and demonstrable sclerotic bodies in only 42.8% cases [13]. Unlike them, we could demonstrate very characteristic sclerotic bodies both in KOH wet mount and stained smears in all five positive cases of present study [Table/Fig-1]. Like ours, they also found lower extremities to be affected more frequently. All positive cases in the present study belong to rural area with profession of agriculture or other field works. Majority gave history of trauma in the adjoining area of body. Likewise similar socioeconomic conditions and history have been cited by the study-group of Kerala [13] and Thailand [12]. But cases reported by De et al., and that of Park SG, Korea had no such history of trauma [10,11]. We have included only the confirmed cases showing either brown sclerotic body in tissue sections by KOH mount or stained smear and/ or growth of causative fungus in multiple test tubes repeatedly from clinical samples. We excluded structures which did not undoubtedly resemble sclerotic bodies. And clinical samples from cases, which showed growth only in one test tube for a single episode, were also not taken into account.
Chromoblastomycosis has many other clinically mimicking lesions as found in our present study viz. lupus vulgaris, atypical mycobacterial infections, hypertrophic lichen planus, hypertrophic DLE plaque, wart, fixed cutaneous sporothricosis, zygomycosis [Table/Fig-3]. Three lesions were diagnosed as atypical mycobacterial infections based on ZN staining features of wound scrapings, culture characteristics on LJ media and MPT-64 antigen testing immune-chromatographic assay. Two cases, both presented with facial lesions were diagnosed as lupus vulgaris and responded well to anti-tubercular therapy. A similar case has been reported from Himachal Pradesh that showed bilaterally symmetrical non-healing facial lesion, initially suspected as chromoblastomycosis has been diagnosed as lupus vulgaris and confirmed by TB-PCR [14]. Another two lesions of our study, one on the left leg and the other on the right foot were diagnosed as hypertrophic lichen planus. Both the lesions were slow growing, non-itchy and verrucous in nature. They didn’t show any sclerotic body nor revealed any fungal growth on culture, instead, showed epidermal hyperplasia, papillomatotosis and lichenoid changes in Haematoxylene & Eosin staining (H&E). A group from western Maharashtra reported a case of chromoblastomycosis on face with similar presentation, which was initially suspected and treated as lichen planus [15]. Rarely fixed cutaneous sporotrichosis can mimic verrucous lesions of chromoblastomycosis. In our study, solitary verrucous lesion on trunk that developed following an injury from unknown vegetable matter was found to be that of fixed cutaneous sporotrichosis. The sample from that lesion showed no fungal element on KOH mount and in calcofluor white staining or it revealed any significant finding on PAS and Gomori’s methanamine silver staining. But in culture, typical moist off-white to cream coloured colony appeared after five days of incubation at 250C, that turned into brownish in another 10 to 12 days. In LPCB wet mount typical twisted rope appearance with petal like small oval conidia were found, summarily suggestive of the growth of Sporothrix schenckii. The patient responded well to the treatment with saturated solution of KI (SSKI) and oral Itraconazole. We found a female diabetic patient with typical cicatrizing lesion on her right cheek following injury mimicking chromoblastomycosis in this present study. Sample from her lesion showed branching aseptate fungal hyphae in both KOH wet mount and calcofluore white staining examination and on culture, it showed fungal growth identified as Mucor raecemosus. Recently a group from Karnataka reported a very unusual case of primary cutaeneus mucormycosis by Rhizomucor varibilis on face, which was previously misdiagnosed as chromoblastomycosis, in a non-diabetic male patient [16]. Blastomycosis is another mimicking lesion though not encountered in current study but has been reported from this region by Maiti P.K. et al. The case showed growth of fluffy white colonies [17]. Therefore a strong degree of clinical suspicion is required to reach to the diagnosis. Else, under detection occurs due to common mimicking lesions. Diagnostic challenges have further been fortified by non detection or unavailability of hallmark feature/s like sclerotic bodies in the clinical sample in first one or two occasions, observers may miss the findings or the patients may be lost in follow up. Present study faced all the three problems to some extent.
At times, prompt laboratory diagnosis is arrived by observing plenty of typical sclerotic bodies from samples of scraping materials from early flat lesions whereas the same may be achieved with great difficulties by demonstrating a few sclerotic bodies only from deep biopsy tissues of warty lesions. So without examination of sufficient suitable materials diagnosis of chromoblastomycosis cannot be ruled out. Optimum mycological diagnosis is sometimes further complicated by frequent appearance of growth of contaminant dematecious fungi in absence of demonstrable tissue form by microscopy. Histopathological diagnosis alone sometimes may be doubtful by presence of some small brown disorganized structures, particularly in superficial layers of skin with features of ‘transepithelial elimination’. Due to paucity of fungal elements in lesions, demonstration of histopathological hallmark structure is considered as confirmatory. On the other hand, their absence cannot rule out the condition. Clinical and/or empirical therapeutic responses sometimes help to rule out mimicking clinical conditions. Though some of these are very poorly sensitive to laboratory testing such as sporotrichosis, lupus vulgaris etc.
Because of diversity of causative fungal elements, bio-availabity of drugs to the affected sites and varied clinical presentations, no uniform treatment recommendation are applicable for all cases. However in all of our cases, initially we tried treatment with oral Itraconazole for three or more months [Table/Fig-2]. In two cases SSKI was given to the patients. Considering the low socio-economic status of the patient, therapy with SSKI was started in one case of progressive lesion but was in-effective, hence, supplemented with oral itraconazole followed by debridement under coverage of itraconazole. Then only we got good response. However, Nair P.S found a knee jerk response with SSKI after around two weeks of treatment in a similar type of lesion [18]. The second one started with oral Itraconazole, followed by inclusion of SSKI in the treatment regimen in the face of dissatisfactory response after three months with good response. Two cases required surgical debridement under cover of antifungal therapy (oral Itraconazole) with good response [Table/Fig-2]. Success of treatment depends on early evaluation of lesions. Therapeutic outcome varies with individual. Therapies can be tried in various combinations and permutations and similar management protocols can show variable results which are related to the causative agent and their susceptibility to specific antifungal and disease severity. Modalities have to be matched with individual tolerance and affordability by the patient. For localized lesions, SSKI is a cheap and efficacious agent and has been used by us successfully. However, after systemic antifungal therapy for a judicious period (3-4 months), if the lesion/disease shows sign of non-responsiveness, the patient may require surgical debridement under coverage of antifungal therapy with or without cauterization of base. This modality of treatment has often been proved to be useful as per our experience.
Limitation
Only a few authentic cases of chromoblastomycosis were reported from eastern India, that too mostly from hilly areas. So, sufficient number of cases are not expected from plain region like ours. Therefore, based on only a few number of suspected cases we had to carry out the study.
Conclusion
Chromoblastomycosis is an infrequently occurring condition in this region (0.007% per year). The diagnosis is complicated by various overlapping clinical and laboratory features of different infective and non-infective conditions. High degree of clinical suspicion, early diagnosis by microscopy and/or culture and prompt institution of therapy is rewarding. Oral Itraconazole is a good option for treatment to start with. A larger and vivid study with more number of cases has to be executed to draw the perfect problem statement of this chronic subcutaneous mycosis in this region of India.
*indicates [Table/Fig-6]
† - figures in the parentheses indicate number of cases