Introduction
Different topical formulations of diclofenac have varying skin penetration profile. Recent advances in science and technology has led to the development of many new formulations of drugs for topical drug delivery. One such technological development has led to the innovation of Dynapar QPS, a novel, non-aqueous, quick penetrating solution (QPS) of diclofenac diethylamine.
Aim
This study was aimed to measure the total exposure from the drug penetrating the skin in healthy human subjects and comparing the relative systemic bioavailability of Dynapar QPS® with diclofenac emulgel.
Materials and Methods
A 200 mg of diclofenac from either Dynapar QPS® (5 ml) or emulgel (20 g) was applied on back of subject as per the randomisation schedule. Blood samples were collected up to 16 hours post drug application. Plasma concentration of diclofenac was measured by pre-validated HPLC method. Pharmacokinetic (PK) parameters like Cmax, Tmax, t1/2, AUC0-t, AUC0-∞, and Kel, of diclofenac were determined for both the formulations.
Results
Mean Cmax after administration of Dynapar QPS® and diclofenac emulgel were 175.93 and 40.04 ng/ml, respectively. Tmax of diclofenac was almost half with QPS compared to emulgel (5.24 hrs versus 9.53 hrs respectively). The mean AUC0–t and AUC0-∞ after administration of Dynapar QPS® was higher as compared to diclofenac emulgel (AUC0–t: 1224.19 versus 289.78 ng.h/ml, respectively; AUC0-∞: 1718.21 versus 513.83 ng.h/ml, respectively). None of the subject experienced any adverse event during the study.
Conclusion
The results indicate an enhanced penetration and subsequent absorption of diclofenac from Dynapar QPS® as compared to diclofenac emulgel. Higher penetration is likely to translate into better pain relief in patients.
Gel, Pharmacokinetics, Quick penetrating solution (QPS), Topical drug
Introduction
Skin, the largest organ, is a formidable barrier to the passage of substances into and out of the body. Skin is composed of three primary layers, the outer epidermis, middle dermis and the hypodermis. The epidermis consists of multiple strata (layers). Stratum corneum, the outermost layer of the skin, is where all of the skin’s barrier property resides. It functions as a physical, environmental and microbial barrier, protecting from external insult and maintaining homeostasis. In order to use the skin as a route for topical drug delivery, this barrier must be overcome. Ideal characteristics of a drug to efficiently penetrate the skin is that the drug molecule should be small in size (<500 Daltons) [1], relatively neutral and lipophilic in nature. A limitation of commonly available topical formulations of drugs is their inability to effectively penetrate the barrier of stratum corneum.
Several studies have reported that, diclofenac is a good NSAID candidate for topical formulations [2]. Diclofenac is the first NSAID approved for topical use in osteoarthritis therapy [3]. Diclofenac is an extensively used NSAID with strong analgesic effect. It is a small molecule (molecular weight of 296.14 Dalton) having a short half-life of 2 hours. Theoretically, this makes diclofenac a suitable molecule for topical formulations. But in clinical practice, transdermal penetration of diclofenac has been found to be variable [4,5]. Only 10% of diclofenac from currently available topical formulations is biologically available [6]. Christopher et al., reported that even nano-particulate based, topical drug delivery systems, cannot penetrate beyond the superficial layers of the barrier [7]. Several strategies have been employed to overcome the problem of low permeability through the skin [8,9]. A popular approach is to use penetration enhancers. These agents partition into the skin, and interact with the constituents of stratum corneum to induce a temporary, reversible increase in skin permeability [10]. An advanced topical formulation with efficient penetration enhancers can make a huge difference in skin permeation of drug molecules.
Dynapar QPS, manufactured by Troikaa Pharmaceuticals Ltd, India; is a novel, non-aqueous, topical formulation of diclofenac diethylamine solubilised in a patented QPS base, which provides quick and comprehensive penetration of drug through the skin. The QPS is a patented technology providing a platform for enhanced penetration of drug into the deeper tissues. Since this novel formulation provides significantly higher transdermal penetration, it quickly relieves musculoskeletal pain and reduce inflammation [11]. A diclofenac formulation with a high degree of skin permeation could be useful in the treatment of inflammatory and painful musculoskeletal conditions.
Higher systemic bioavailability can be correlated with the extent of skin penetration of diclofenac from novel formulation. To evaluate the skin permeation of this novel topical formulation, the plasma levels of diclofenac achieved from Dynapar QPS® and Diclofenac gel were estimated. Several methods have been reported for determination of diclofenac including gas chromatography-mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC) and LC–MS–MS in human plasma and other biological fluids [12].
In the present study, we have used a simple HPLC with UV method for determination of diclofenac in human plasma. The developed method was validated for linearity, stability, precision, accuracy, and sensitivity parameters according to International Conference on Harmonization (ICH) guidelines. The advantages of the present bioanalytical method include simple and single step extraction procedure and short run time. At the same time, the method was efficient in evaluating pharmacokinetic profile from therapeutic doses of diclofenac after topical administration in healthy human subjects [12].
This study was aimed to measure the total exposure from the drug penetrating the skin in healthy human subjects and comparing the relative systemic bioavailability of Dynapar QPS® with diclofenac emulgel.
Materials and Methods
Subjects
Total 18 (nine in each group) healthy, non-smoking, adult male volunteers (mean age±SD, 32.70±5.64) were enrolled in the study based on normal findings from laboratory investigations. The mean Body Mass Index (BMI) of the subjects was 22.49 ± 1.93 kg/m2. The study protocol was approved by the Institutional Ethics Committee and the study was conducted in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki. Prior to any screening procedure, written consent was obtained from each subject participating in this study after adequate explanation of the aims, methods, objective, and potential hazards of the study.
Main exclusion criteria were: hypersensitivity to any allergens, history or presence of cardiovascular, respiratory, hepatic, renal, gastrointestinal, endocrine, immunological, dermatological, neurological, psychiatric disease or any other systemic disease; regular alcohol intake or drug abuse within the past one-year; smoking (more than 10 cigarettes per day) or consumption of tobacco products. The volunteers were asked to abstain from taking any medication, all type of tobacco products and alcohol, throughout the study period.
The study was conducted during December 2010 to January 2011 at B. V. Patel PERD Centre, Sarkhej-Gandhinagar Highway, Thaltej, Ahmedabad, Gujarat, India.
Study Drugs
Dynapar QPS® (Diclofenac diethylamine non-aqueous topical solution 4%; Troikaa pharmaceuticals Ltd., India) was used as test and Emulgel 1% (diclofenac sodium emulgel 1%) was used as reference drug.
Study Design
This was an open label, balanced, randomized, two-treatment, two-sequence, two-period, single dose, crossover comparative bioavailability study.
Subjects were housed in the clinical facility 10 hrs before administration of the dose and were discharged 24 hours post dose. As per the randomisation sheet, 200 mg of diclofenac from either diclofenac QPS (5 ml) or emulgel (20 g) was applied on the back of each subject. The wash-out time between period I and period II of the study was 7 days. Subjects were kept in prone position for 2 hours and were not allowed to wear their shirt till 4 hours.
All subjects received standard light breakfast one hour prior to dosing; and lunch, snacks (fruit juice and biscuits) and dinner 4, 8 and 12 hours post dosing. During housing, all meal plans were similar for both the periods.
Blood Sampling
Blood samples (6 ml aliquots including 0.2ml discarded heparinised blood) were collected in labeled vacutainer tubes before dosing and at 15, 30, 45 minutes, 1,2,3,4,5,6,7,8,9,10,11,12,14 and 16 hours after dosing. Centrifugation of blood samples was carried out at 4°C and 4000 rpm for 7 minutes to separate plasma. Plasma samples were stored in deep freezer at –20 ± 5°C for interim storage and finally stored at –70 ± 5°C until analysed.
Determination of Diclofenac in Human Plasma Sample preparation
Samples were prepared by vortex mixing 50 μl Mafenamic Acid (internal standard) to 500 μl plasma, followed by addition of 50 μl of 6% trichloroanisole (TCA). This was again vortex mixed briefly and extracted with 6ml Dichloromethane. The organic fraction was separated, evaporated and reconstituted in 100 μl mobile phase. A 70μl aliquot of this extract was injected onto HPLC column.
HPLC Conditions and Sample Analysis
Analytes were separated using a reverse phase Grace Vydac C18 (250 x 4.6 mm, 5 micron) analytical column and the UV detector set at 282 nm. Auto sampler temperature was ambient. The mobile phase consisted of 65 % (v/v) buffer 10 mM potassium di hydrogen orthophosphate in water, pH 6.30 adjusted with dilute potassium hydroxide solution.) and 35% acetonitrile. The flow rate was 0.8 ml.min-1. The lower limit of quantification of diclofenac was 5 ng.ml-1. The intra and interday precision and accuracy for low (20 ng/ml), medium (400 ng/ml) and high (850 ng/ml) concentrations of diclofenac were < 15% and < 20% (at lowest limit of quantification).
Pharmacokinetic Analysis
The maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) were determined for individual plasma concentration time profiles. The slope of the terminal log-linear portion of the concentration-time profile was determined by least-squares regression analysis and used for estimation of elimination rate constant (Kel). The terminal half life (t1/2) was calculated by the formula 0.693/kel. The AUC0-t from time zero to the last quantifiable point (Ct) was calculated using the trapezoidal rule and the extrapolated AUC from Ct to infinity AUC0-∞ was determined as Ct/Kel. The area under the plasma concentration-time from zero to infinity AUC0-∞ was calculated as the sum of the AUC0-t and the ratio of the last measurable concentration to the elimination rate constant.
Results
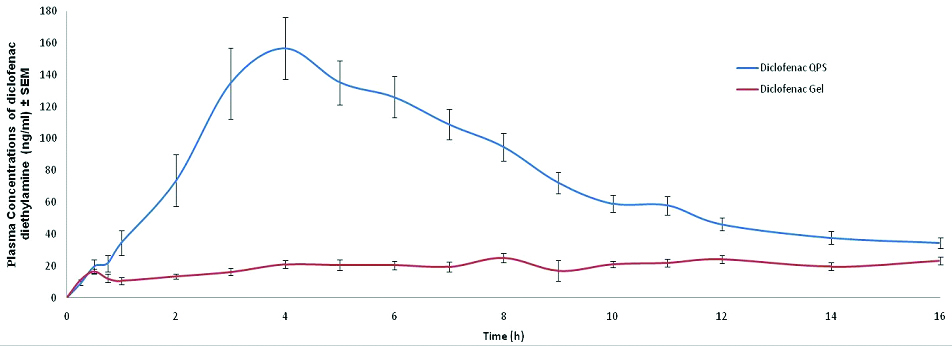
The mean pharmacokinetic parameters and mean plasma concentrations versus time profiles of diclofenac from both formulations are shown in [Table/Fig-1,2] respectively. The mean Cmax of diclofenac after application of Dynapar QPS® was higher as compared to emulgel (175.93 versus 40.04 ng/ml respectively, p=0.0001). Tmax was shorter with QPS (5.24 h) as compared to emulgel (9.53 h, p=0.0011). Extent of absorption of diclofenac, as determined from mean AUC0–t values, were 1224.19 and 289.78 ng.h/ml and AUC0–∞ values were 1718.21 and 513.83 ng.h/ml respectively from Dynapar QPS® and emulgel. The mean apparent t1/2 of Dynapar QPS®(21.23 h) was longer than mean t1/2 of diclofenac emulgel (15.42 h), p=0.3498. In this study, no adverse event, premature withdrawals, replacements or any serious adverse events was observed. There was only one drop out in this study due to no show in period II. All the laboratory results, vital signs and post study physical examinations were in the normal range and did not indicate any clinical abnormality.
Summary of pharmacokinetic parameters of diclofenac diethylamine in plasma, following administration of the reference and test formulations
| Parameter | Cmax(ng/ml) | Tmax(hrs) | AUC0-t(ng.h/ml) | AUC0-∞(ng.h/ml) | t½(hrs) | Kel(h-1) |
|---|
| T | R | T | R | T | R | T | R | T | R | T | R |
|---|
| MEAN | 175.93 | 40.04 | 5.24 | 9.53 | 1224.19 | 289.78 | 1718.21 | 513.83 | 21.23 | 15.42 | 0.08 | 0.07 |
| SD | 89.49 | 26.62 | 2.59 | 4.2 | 445.69 | 139.74 | 740.58 | 395.66 | 19.99 | 16.12 | 0.1 | 0.04 |
| SEM | 20.53 | 6.11 | 0.59 | 0.96 | 102.25 | 32.06 | 169.9 | 90.77 | 4.59 | 3.7 | 0.02 | 0.01 |
| N | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 |
| % CV | 50.9 | 66.5 | 49.4 | 44.1 | 36.4 | 48.2 | 43.1 | 77 | 94.2 | 104.5 | 128.7 | 56.7 |
| p-value | 0.0001 | 0.0011 | 0.0001 | 0.0001 | 0.3498 | 0.7044 |
R: Reference formulation (emulgel); T: Test formulation (QPS); Data were analysed by unpaired t-test
Linear plot of mean plasma concentrations (ng/ml) versus time (h) profile of diclofenac diethylamine for test and reference formulation.
Discussion
Higher mean Cmax of diclofenac produced by Dynapar QPS® as compared to diclofenac emulgel, indicates higher and deeper penetration of diclofenac through the stratum corneum into the local tissue. Shorter Tmax from QPS compared to emulgel suggests rapid penetration of diclofenac into the deeper layers of the skin resulting in faster onset of clinical response. Higher penetration of Dynapar QPS® compared to gel, through the stratum corneum has been established earlier in a dermal microdialysis study [13]. Pradhan et al., observed that Dynapar QPS® provides better pain relief as compared to diclofenac gel [11] Better pain relief may be explained as a result of better penetration and adequate therapeutic levels of diclofenac in tissue from Dynapar QPS® compared to gel.
Lower systemic, higher local tissue and skeletal muscle concentrations of diclofenac after topical application compared to oral administration in healthy volunteers, were reported by Kienzler et al., [14]. The systemic levels of topical formulation of diclofenac are reported to be 50 times lower as compared to the systemic levels produced after oral administration of diclofenac [15]. Therefore, the systemic adverse events after topical diclofenac would be less as compare to the oral diclofenac. Several studies have demonstrate that, because of low systemic concentrations, topical NSAIDs have reduced risk of upper gastrointestinal (GI) complications such as gastric and peptic ulcers, and GI symptoms such as dyspepsia, as well as a lack of drug–drug interactions [16].
Higher AUC values indicate a trend towards 4 folds higher bioavailability from Dynapar QPS® as compared to gel. The higher penetration of Dynapar QPS® as expressed by the comparative bioavailability study, establishes that advanced formulation of Dynapar QPS® would provide more efficient and deeper skin penetration and provide early onset of clinical benefits.
Further studies are recommended to measure plasma as well as tissue concentrations after oral administration and topical diclofenac application.
Conclusion
In this comparative bioavailability study, the rate and extent of diclofenac penetration from skin was higher from Dynapar QPS® and thus has higher bioavailability as compared to diclofenac emulgel formulation. This higher topical bioavailability may translate into clinical superiority of Dynapar QPS® over emulgel.
R: Reference formulation (emulgel); T: Test formulation (QPS); Data were analysed by unpaired t-test
[1]. Bos JD, Meinardi MM, The 500 Dalton rule for the skin penetration of chemical compounds and drugsExp Dermatol 2000 9(3):165-69. [Google Scholar]
[2]. Escribano E, Calpena AC, Queralt J, Obach R, Doménech J, Assessment of diclofenac permeation with different formulations: anti-inflammatory study of a selected formulaEur J Pharm Sci 2003 19(4):203-10. [Google Scholar]
[3]. Altman R, Barkin RL, Topical therapy for osteoarthritis: clinical and pharmacologic perspectivesPostgrad Med 2009 121(2):139-47. [Google Scholar]
[4]. Dehghanya P, Mayer BX, Namiranian K, Mascher H, Müller M, Brunner M, Topical skin penetration of diclofenac after single- and multiple-dose applicationInt J Clin Pharmacol. Ther 2004 42(7):353-59. [Google Scholar]
[5]. Nair B, Taylor-Gjevre R, A Review of Topical Diclofenac Use in Musculoskeletal DiseasePharmaceuticals 2010 3:1892-908. [Google Scholar]
[6]. Cevc G, Blume G, New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable carriers, transferosomesBiochim Biophys Acta 2001 1514(2):191-205. [Google Scholar]
[7]. Campbell CSJ, Contreras-Rojas LR, Delgado-Charro MB, Guy RH, Objective assessment of nanoparticle disposition in mammalian skin after topical exposureJ Control Release 2012 162(1):201-07. [Google Scholar]
[8]. Nokhodchi A, Sharabiani K, Rashidi MR, Ghafourian T, The effect of terpene concentrations on the skin penetration of diclofenac sodiumInt J Pharm 2007 335(1-2):97-105. [Google Scholar]
[9]. Jain Manu, Lohare S, Ganesh B, Bari Manoj M, Chavan Randhir B, Barhate SD, Permeation studies of Diclofenac sodium from buffalo ghee as an oleaginous baseDer Pharmacia Lettre 2011 3(5):244-48. [Google Scholar]
[10]. Ammar HO, Ghorab M, El-Nahhas SA, Kamel R, Evaluation of chemical penetration enhancers for transdermal delivery of aspirinAsian journal of Pharmaceutical science 2007 2(3):96-105. [Google Scholar]
[11]. Pradhan CV, Talesara JM, Sharma AB, Panchal VH, Ramanathan S, Patel KR, A Novel Quick Penetrating Solution Of Diclofenac (Topical) For Management Of Acute Musculoskeletal PainInt J Res Med 2013 2(2):103-08. [Google Scholar]
[12]. Yilmaz B, Asci A, Palabiyik SS, HPLC Method for Determination of Diclofenac in Human Plasma and Its Application to a Pharmacokinetic Study in TurkeyJ Chromatogr Sci 2011 49(6):422-27. [Google Scholar]
[13]. Maroo SH, Patel KR, Prajapati V, Shah R, Bagul M, Ojha RU, A comparative dermal microdialysis study of diclofenac QPS versus conventional 1% diclofenac gelInt J Pharm Sci Drug Res 2013 5(3):175-78. [Google Scholar]
[14]. Kienzler JL, Gold M, Nollevaux F, Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteersJ Clin Pharmacol 2010 50(1):50-61. [Google Scholar]
[15]. Brunner M, Dehghanyar P, Seigfried B, Martin W, Menke G, Müller M, Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulationBr J Clin Pharmacol 2005 60(5):573-77. [Google Scholar]
[16]. Mccarberg BH, Argoff CE, Topical diclofenac epolamine patch 1.3% for treatment of acute pain caused by soft tissue injuryInt J Clin Pract 2010 64(11):1546-53. [Google Scholar]