Osteochondroma of Upper Dorsal Spine Causing Spastic Paraparesis in Hereditary Multiple Exostosis: A Case Report
Gaurav Kumar Upadhyaya1, Vijay Kumar Jain2, Rajendra Kumar Arya3, Skand Sinha4, Ananta Kumar Naik5
1 Senior Resident, Department of Orthopaedics, PGIMER & Dr. RML Hospital, New Delhi, India.
2 Chief Medical Officer, Department of Orthopaedics, PGIMER & Dr. RML Hospital, New Delhi, India.
3 Associate Professor, Department of Orthopaedics, PGIMER & Dr. RML Hospital, New Delhi, India.
4 Assistant Professor, Department of Orthopaedics, PGIMER & Dr. RML Hospital, New Delhi, India.
5 Associate Professor, Department of Orthopaedics, PGIMER & Dr. RML Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vijay Kumar Jain, Chief Medical Officer, Department of Orthopaedics, Dr Ram Manohar Lohia Hospital, Baba Kharak Singh Marg, New Delhi-110001, India.
E-mail: bedirrecep@gmail.com
Osteochondroma of the spine is rare. It may present in solitary or multiple form (hereditary multiple exostoses). Herein, we report a case of an 18-year-old male who was diagnosed with thoracic osteochondroma, originating from the D4 vertebra with intraspinal extension and spinal cord compression in hereditary multiple exostosis. The patient was managed with surgery. Complete tumour excision was done to relieve cord compression and recurrence. Postoperatively the patient’s symptoms were improved. At 2.5 year follow-up patient is doing well without any recurrence.
Myelopathy, Spine, Thoracic
Case Report
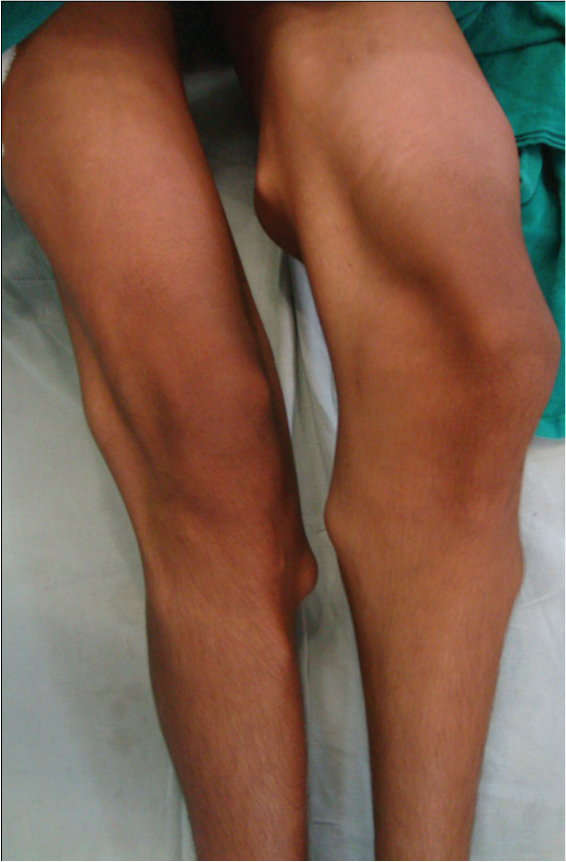
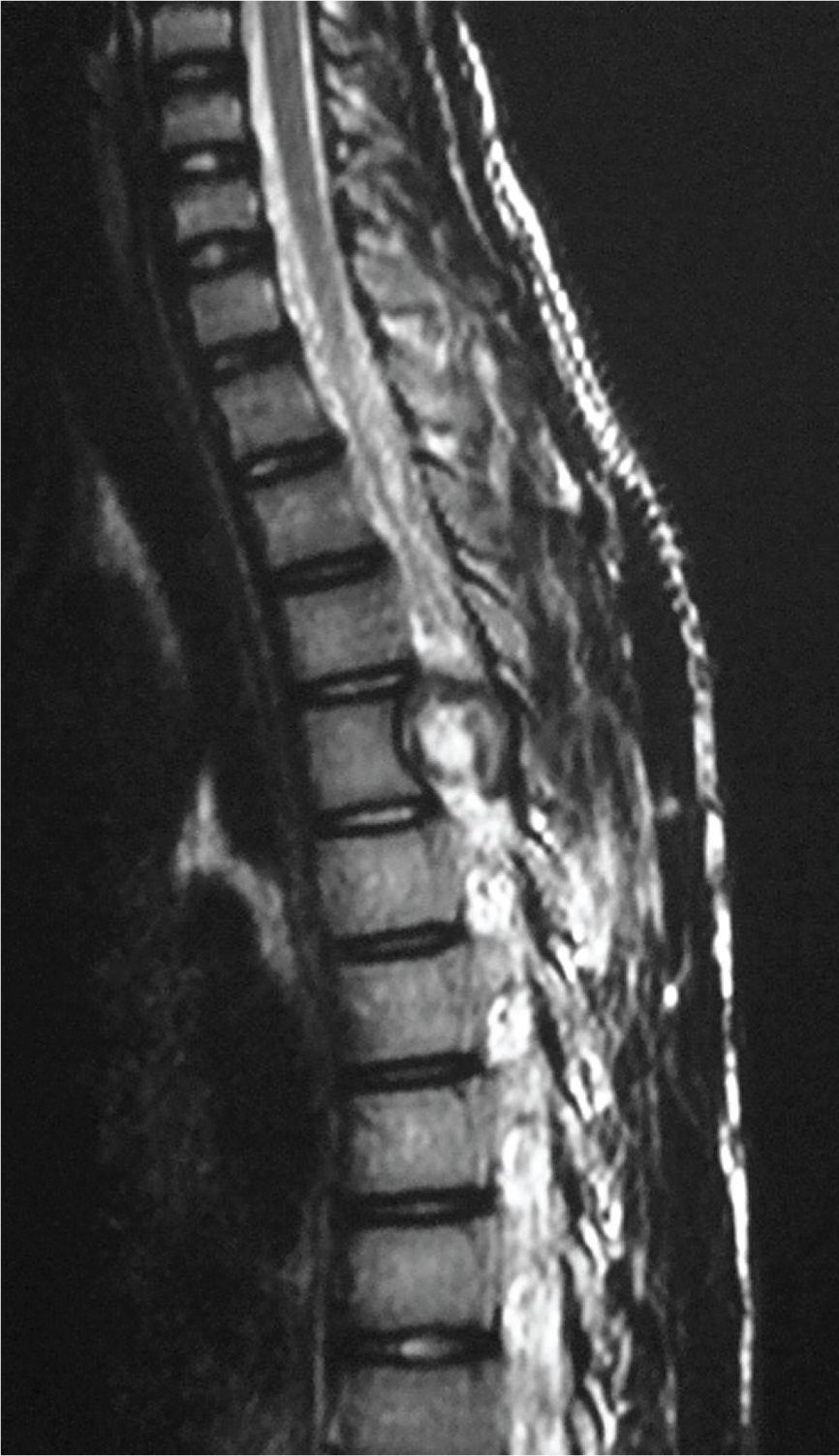
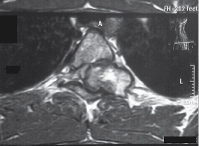
An 18-year-old male patient presented in outpatient department with complaints of multiple bony hard swellings in the arms, thighs and legs since the past 5 years and inability to walk due to weakness of both the lower limbs since the past 5 months. The complaint of weakness in both the lower limbs was gradually progressive over a period of 5 months. There was no history suggestive of bladder or bowel involvement. Patient had a positive family history with multiple osteochondromas in his younger sibling. There was no history of trauma, fever, and weight loss. On physical examination multiple, non-tender, fixed, bony hard swellings of varying sizes were present in the extremities [Table/Fig-1]. The local examination of spine was normal. The neurological examination revealed spastic paraparesis with reduced sensation below D6 dermatome on both sides. There was weakness, decreased pinprick sensation, and hyperreflexia of his lower extremity. Positive Babinski response was also elicited. Bladder and bowel functions were intact. Radiograph of the upper dorsal spine revealed exophytic bony mass overlying D4 vertebra more on the right side. Magnetic resonance imaging of the whole spine showed 3cm x 1cm bony mass arising from the right pedicle of the 4th thoracic vertebra involving the contiguous lamina, spinous process and extending from vertebral foramen to vertebral canal compressing the spinal cord. There was marked cord compression seen at this level without change in signal intensity [Table/Fig-2,3]. Radiographs of the extremities too revealed multiple mass arising from the ends of the involved bones. Excision of mass in the D4 vertebra was undertaken by posterior approach. Tumour mass was removed including lamina and pedicle of the 4th thoracic vertebrae on the right side along with facetectomy on the right side. The excised mass was sent for histopathological examination which confirmed the diagnosis of osteochondroma. The patient was given choice to remove other swellings of the body for cosmetic correction but he had not given consent for any other surgeries. Postoperatively the tone of bilateral lower limbs became normal. The weakness and numbness improved. The patient was able to ambulate on his own without support at 3 months after surgery and there was full neurological recovery after 1 year postoperatively. At 2.5 years follow up period patient is doing well and is walking without support.
Clinical photograph showing multiple bony masses in a patient with hereditary multiple exostoses
MRI upper dorsal spine sagittal image reveals mass in vertebral canal compressing the spinal cord at D4 level
MRI upper dorsal spine axial image exophytic bony mass overlying D4 vertebra more on the right side arising from the right pedicle of the D4 vertebra involving the contiguous lamina, spinous process and extending from vertebral foramen into spinal canal
Discussion
Osteochondromas are the most common benign bone tumours presented as solitary (90%) or multiple lesions (10%) [1]. They are frequently located in the long and flat bones. The involvement of the spine is very rare and comprises only 1.3%- 4.1% of all osteochondromas of the spine [2]. With the best of our knowledge there are about 27 cases of thoracic vertebral exostosis in patients with Hereditary Multiple Exostosis (HME) has been described in the English literature [Table/Fig-4] [1,3–26]. The multiple osteochondromas are present in HME. HME is a genetic disorder with autosomal dominance pattern and are associated with mutations in tumour suppressor genes EXT1or EXT2orEXT3 located on chromosome 8q, 11p and 19p respectively [27]. OC are considered as developmental lesions rather than true neoplasms. Although aetiologically not clear, OCs are originated from the separation of epiphyseal growth plate cells followed by herniation through the periosteum adjacent the growth plate [26]. The common site of OC are long bones, particularly around the knee joint, the upper humerus, flat bones especially pelvis, scapula, and ribs. It rarely occurs in the vertebrae and accounts for 1-4% of cases [2]. The vertebral OC are more common in younger male patients as seen in our case [21].
Previously reported cases of thoracic vertebral osteochondromas
| Author | Year | Age | Sex | Familyhistory | Level | Origin | Presentingcomplaint | Surgery | Outcome | Follow up | Remark |
|---|
| Cannon [3] | 1954 | 23 | F | Yes | D 10 | NR | Weaknesstinglingnumbness | laminectomy | Good | NR | Nocomplication |
| Larsonet al., [4] | 1957 | 33 | M | No | D 3 | CVJ | Paraplegia | yes | Good | NR | _ |
| Decker& Wei [5] | 1969 | 15 | M | No | D10 | CVJ | Parapareses | yes | Good | NR | AssociatedCerebellarastrocytoma |
| Blaauw [6] | 1975 | 48 | M | NR | D 1 | CVJ | NR | yes | Good | NR | - |
| Twerskyet al., [7] | 1975 | 53 | M | No | D 5 | CVJ | NR | yes | Worsened | NR | Associated costalosteochondroma |
| Becker &Epstein [8] | 1978 | 17 | M | NR | D 2 | CVJ | NR | yes | Good | NR | - |
| Ho & Lipton[9] | 1979 | 58 | F | Yes | D1- D2 | Laminainferoposterioraspect | 15-year gradualProgressive weakness,numbness,bladder involve | laminectomy | Poor | 12 Mo | No recovery |
| Old &Triplett [10] | 1979 | 21 | F | Yes | D 3 | CVJ | NR | yes | Good | NR | _ |
| Buur &Mørch [11] | 1983 | 33 | M | Yes | D 4 | Pedicle | Spastic paraparesis | Laminectomy | Good | 7 Mo | _ |
| Moriwakaet al., [12] | 1990 | 9 | M | NR | C7- D1 | Pedicleand VB | Pain thigh,couln’t walk | Laminectomy,Facetectomy | Improved/completerecovery Good | 3 Mo | _ |
| O’Brienet al., [13] | 1994 | 14 | F | NR | D9-D10,D 12 | PedicleD9, D10ExtracanalicularD12Intracanalicular | Decreased senastionwith paresthesisa,spasticparaparesiswith bladderinvolvement | Wide laminectomyT11-l1,decompressionand posteriorfusin | Good | 1 Mo | _ |
| Quiriniet al., [1] | 1996 | 24 | M | NR | D 8 | VB endplate | Difficulty in walking,numbness | yes, Excision | Good | NR | _ |
| Govender &Parbhoo[14] | 1999 | 14 | F | NR | D 8 | Neural arch | Weakness of bothlower limbs andurinary incontinence | Posteriordecompression | Good | 3 Mo | MisdiagnosedTuberculosis |
| Mermeret al., [15] | 2002 | 15 | M | Yes | D 5 | VB | Weakness ofright lower limb | Anterior decompressionof T4–5 with anterior andposterior spinal fusion | Good | 6 Mo | one or two clonusbeats Remained |
| Faik et al.,[16] | 2004 | 17 | M | NR | D2-D3 | Pedicle/VB | spastic paraparesis | decompressionlaminectomy | Good | NR | No complication |
| Besset al., [17] | 2005 | 11 | F | Yes | D 5 | VB | Ataxia, Hyperreflexia | Observation | Good | 29Mo | No complication,treated conservative |
| Roachet al., [18] | 2009 | NRNRNR | MNRM | NRNRNR | D 9 | NRNRVB | QuadriplegiaProgressiveweakness& AtaxiaParaplegia | ExcisionExcisionNo | Partially resolvedGoodPoor | NRNRNR | Associatedcervical lesion |
| Ezraet al., [19] | 2010 | 4 | M | Yes | C7- D1 | Lamina,spinousprocess | Pain in neck & B/LLeg Difficulty inwalking urinaryincontinence | LaminectomyAnd Excision | Improved | NR | Residual deficitsincluded right armand hand weaknessand a mild limp |
| Gunayet al., [20] | 2010 | 36 | F | YES | D 12 | Pedicle | Pain, Weakness,Numbness | Excision Laminectomywith Instrumentation | Good | 44 Mo | slight hypoesthesiaon T 11 Dermatome |
| Lotfiniaet al., [21] | 2010 | 31 | M | YES | D 8 | Facet | B/L Paresthesia,Paraperesis | Laminectomy withInstrumentation | Poor | NR | Partial Improvement |
| Tianet al., [22] | 2011 | 16 | M | YES | D 6 | VB endplate | progressiveweaknessand ascendingnumbness | Laminectomywith fusion | Good | 12Mo | _ |
| Zaijunet al., [23] | 2013 | 16 | M | YES | D5-D6 | SP & TP | Paraplegia,Hypesthesia | yes | Good | 9 Mo | No complication |
| Bariet al., [24] | 2012 | 16 | M | NO | D9-D12 | NR | Restrictedmovementsand urinaryincontinence | Surgery | Good | NR | No complication |
| Al Kaissiet al., [25] | 2013 | 9 | M | NR | D3-5 | Pedicle | NR | yes | | NR | _ |
| Calvoet al., [26] | 2013 | 9 | M | NR | D 3 | Pedicle | Inability to walk | Laminectomyinstrumentation | Good | 3Mo | _ |
| Presentcase | 2014 | 18yr | M | YES | D 4 | Pedicle,lamina | Paraperesis | Excision | Good | 2.5 years | _ |
Abbreviations: * CT-Computerized tomography; CVJ- Costovertebral junction; MRI -Magnetic resonance imaging; HME- hereditary multiple exostoses; NR- not reported; Mo - month; OC-osteochondroma; SP- spinous process; TP- Transverse process; VB- vertebral body
About 1% and 4% of solitary osteochondromas and 7% to 9% in hereditary multiple exostoses develop a spinal lesion [26]. The spinal involvement and neurological complications in multiple osteochondromas is higher than solitary variety [27]. In HME, thoracic and lumbar vertebrae are more commonly affected, while in solitary type cervical spine is commonly involved [16]. The involvement of sacrum is rare in both the types. A review of English literature revealed about 27 cases of thoracic myelopathy due to spinal exostosis in HME [Table/Fig-4]. Mean age of the patients was 22.5 years. Nineteen patients were male while seven patients were female. D5 vertebrae (19%) were found to be most commonly affected.
Though any part of vertebrae can be involved, the posterior arch is the most commonly affected [23]. In present case pedicle and lamina both were involved. Patients may present with back pain, cosmetic deformity and or a palpable mass. Very rarely vertebral OC may extend into the spinal canal causing cord compression and present with neurological compromise as occurred in our patient. Myelopathy is predominantly seen with multiple OCs [16].
On plain radiographs, vertebral OC is seen as bony outgrowth continuous with cortex of the bone from where it was originated. The bone marrow of the mass is continuous with the normal bone. The vertebral OCs are often small, sessile and easily missed on radiography. Computed tomography (CT) is useful to demonstrate spinal OCs which are small and have narrow stalk. In addition it is the best method to detect marrow, cortical continuity of vertebral OC though it was not done in our case. MRI of the whole spine should be performed in these cases to look for skip lesions or other masses and relation of vertebral OC to the surrounding structures. MRI shows the extent of cord compression as well as also assesses the size of cartilaginous component to rule out malignant degeneration of vertebral osteochondroma. MRI helps in early identification and planning surgery in these cases [16,23]. Similarly early MRI and surgery prevent permanent neurologic deficits as in our case. We suggest that screening MRI of the whole spine should be used in HME patients for early diagnosis of vertebral OC before the development of neurological deficit.
Grossly vertebral OC is seen as irregular bony mass with the gray white cartilaginous cap. Histologically, it has hyaline cartilages and mature bony spurs [6]. Complications of vertebral OC can include adjacent vascular and neural compromise, fracture, osseous deformity, bursa and malignant change. The malignant transformation in HME is more common than solitary osteochondromas [20]. Early diagnosis should be made in these cases if there is neurological compromise for good results. Asymptomatic vertebral OC can be left as such and patients should be followed up. Review of literature showed surgery was done in majority of cases and resulted in good results in most patients [3–6,8,10–20,22–26]. Surgical excision and decompression of spinal canal vertebral OC is required in these cases. Similarly the decompression and excision of the mass was done in our case. Recurrence can occur due to incomplete excision therefore complete "en bloc" resection is recommended. These patients should be followed up for long duration as relapse or malignant transformation may occur after surgery.
Conclusion
Though spine is very rare site for osteochondroma, high index of suspicion is required to diagnos it. A vertebral OC should be excluded in all patients with hereditary multiple exostosis who presents with spinal pain and neurological deficit. Surgical intervention generally has good outcome.
Abbreviations: * CT-Computerized tomography; CVJ- Costovertebral junction; MRI -Magnetic resonance imaging; HME- hereditary multiple exostoses; NR- not reported; Mo - month; OC-osteochondroma; SP- spinous process; TP- Transverse process; VB- vertebral body
[1]. Quirini GE, Meyer JR, Herman M, Russell EJ, Osteochondroma of the thoracic spine: an unusual cause of spinal cord compressionAJNR Am J Neuroradiol 1996 17(5):961-64. [Google Scholar]
[2]. Pazzagilia UE, Perdotti L, Beluffi G, Monafo V, Savasta S, Radiographic finding in hereditary multiple exostoses and a new theory of the pathogenesis of exostosesPediatr Radiol 1990 20(8):594-97. [Google Scholar]
[3]. Cannon JF, Hereditary multiple exostosesAm J Hum Genet 1954 6(4):419-25. [Google Scholar]
[4]. Larson NE, Dodge HW, Rushton JG, Dahlin DC, Hereditary multiple exostoses with compression of the spinal cordProc Staff Meet Mayo Clin 1957 32(25):729-34. [Google Scholar]
[5]. Decker RE, Wei WC, Thoracic cord compression from multiple hereditary exostoses associated with cerebellar astrocytoma. Case reportJ Neurosurg 1969 30(3):310-12. [Google Scholar]
[6]. Blaauw G, Osteocartilaginousexostosis of the spine, in Vinken PJ, Bruyn CW (eds)Handbook of Clinical Neurology 1975 Vol 19AmsterdamNorth Holland Publishing:313-19. [Google Scholar]
[7]. Twersky J, Kassner EG, Tenner MS, Camera A, Vertebral and costal osteochondromas causing spinal cord compressionAm J Roentgenol Radium TherNucl Med 1975 124(1):124-28. [Google Scholar]
[8]. Becker MH, Epstein F, Case report 77Skeletal Radiol 1978 3:197-99. [Google Scholar]
[9]. Ho SU, Lipton HL, Hereditary multiple exostoses with myelopathyArch Neurol 1979 36(11):714 [Google Scholar]
[10]. Old WL, Triplett JN, Osteochondroma with thoracic cord compression in hereditary multiple exostoses: a case reportVa Med 1979 106(5):302-06. [Google Scholar]
[11]. Buur T, Mørch MM, Hereditary multiple exostoses with spinal cord compressionJ Neurol Neurosurg Psychiatry 1983 46(1):96-98. [Google Scholar]
[12]. Moriwaka F, Hozen H, Nakane K, Sasaki H, Tashiro K, Abe H, Myelopathy due to osteochondroma: MR and CT studiesJ Comput Assist Tomogr 1990 14(1):128-30. [Google Scholar]
[13]. O’Brien MF, Bridwell KH, Lenke LG, Schoenecker PL, Intracanalicularosteochondroma producing spinal cord compression in hereditary multiple exostosesJ Spinal Disord 1994 7:236-41. [Google Scholar]
[14]. Govender S, Parbhoo AH, Osteochondroma with compression of the spinal cord. A report of two casesJ Bone Joint Surg Br 1999 81(4):667-69. [Google Scholar]
[15]. Mermer MJ, Gupta MC, Salamon PB, Benson DR, Thoracic vertebral body exostosis as a cause of myelopathy in a patient with hereditary multiple exostosesJ Spinal Disord Tech 2002 15(2):144-48. [Google Scholar]
[16]. Faik A, MahfoudFilali S, Lazrak N, El Hassani S, Hajjaj-Hassouni N, Spinal cord compression due to vertebral osteochondroma: report of two casesJoint Bone Spine 2005 72(2):177-79. [Google Scholar]
[17]. Bess RS, Robbin MR, Bohlman HH, Thompson GH, Spinal exostoses: analysis of twelve cases and review of the literatureSpine. (Phila Pa 1976) 2005 30(7):774-80. [Google Scholar]
[18]. Roach JW, Klatt JW, Faulkner ND, Involvement of the spine in patients with multiple hereditary exostosesJ Bone Joint Surg Am 2009 91(8):1942-48. [Google Scholar]
[19]. Ezra N, Tetteh B, Diament M, Jonas AJ, Dickson P, Hereditary multiple exostoses with spine involvement in a 4-year-old boyAm J Med Genet A 2010 152A(5):1264-67. [Google Scholar]
[20]. Gunay C, Atalar H, Yildiz Y, Saglik Y, Spinal osteochondroma: a report on six patients and a review of the literatureArch Orthop Trauma Surg 2010 130(12):1459-65. [Google Scholar]
[21]. Lotfinia I, Vahedi P, Tubbs RS, Ghavame M, Meshkini A, Neurological manifestations, imaging characteristics, and surgical outcome of intraspinalosteochondromaJ Neurosurg Spine 2010 12(5):474-89. [Google Scholar]
[22]. Tian Y, Yuan W, Chen H, Shen X, Spinal cord compression secondary to a thoracic vertebral osteochondromaJ Neurosurg Spine 2011 15(3):252-57. [Google Scholar]
[23]. Zaijun L, Xinhai Y, Zhipeng W, Wending H, Quan H, Zhenhua Z, Outcome and prognosis of myelopathy and radiculopathy from osteochondroma in the mobile spine: a report on 14 patientsJ Spinal Disord Tech 2013 26(4):194-99. [Google Scholar]
[24]. Bari MS, Jahangir Alam MM, Chowdhury FR, Dhar PB, Begum A, Hereditary multiple exostoses causing cord compressionJ Coll Physicians Surg Pak 2012 22(12):797-99. [Google Scholar]
[25]. Al Kaissi A, Ganger R, Klaushofer K, Grill F, Spinal exostosis in a boy with multiple hereditary exostosesCase Rep Orthop 2013 2013:758168 [Google Scholar]
[26]. Calvo CE, Cruz M, Ramos E, An unusual complication in a 9-year-old patient with hereditary multiple osteochondromatosisPMR 2013 5(4):348-50. [Google Scholar]
[27]. Lemos MC, Kotanko P, Christie PT, A novel EXT1 splice site mutation in a kindred with hereditary multiple exostosis and osteoporosisJ Clin Endocrinol Metab 2005 90(9):5386-89. [Google Scholar]