Periodontitis is a naturally occurring reflection of chronic low-grade infection and inflammation [1]. It leads to haemodynamic, endothelial, and inflammatory changes. Periodontal inflammation is a probable risk factor for a procoagulant state, which is marked by the propensity of blood to coagulate secondary to an abnormality in the coagulation or fibrinolysis systems [2]. Thus, the concentrations of fibrinogen and fibrin degradation products differ drastically in various periodontal diseases.
D-Dimer is a polymer released during dissolution of the fibrin clot during fibrinolysis [2]. It stimulates the monocyte–macrophage lineage cells to secrete interleukin-1. Elevated concentrations of D-dimer in the plasma are a direct indication of activation of the fibrinolytic system, an indirect indicator that a blood clot has formed somewhere in the body [3–6]. The D-dimer assay is a simple, rapid, and powerful tool to assist clinicians in evaluating low to moderate risk patients for exclusion of deep venous thrombosis without costly imaging procedures.
Materials and Methods
Study Group: Forty systemically healthy adult subjects were selected from among the patients reporting to the Department of Periodontology, Faculty of Dental Sciences, SGT University, Delhi-NCR, India, 2014. The subjects had at least 20 teeth in both jaws and had not received antibiotic therapy in the previous six months or periodontal therapy in the previous 12 months. Subjects not included were:- pregnant women and lactating mothers, subjects with a history of tobacco use, alcoholism, or liver, kidney, diabetes, or salivary gland dysfunction, subjects using any medication possibly affecting the periodontal tissues such as cyclosporine A, calcium channel blockers, and phenytoin in the preceding six months; subjects with a history of antibiotic and non-steroidal anti-inflammatory use in the previous 6 months; and subjects who had undergone organ transplantation or cancer therapy. Four groups were formed: Group I =10 healthy subjects, Group II = 10 with mild periodontitis (clinical attachment level {CAL} 1–2 mm, probing depth {PD} 3–4 mm), Group III = 10 with moderate periodontitis (CAL 3–4 mm, PD 5–7 mm), and Group IV = 10 with severe periodontitis (CAL •5 mm, PD >7 mm) [13] [Table/Fig-1]. The study was approved by the institutional review board. Each subject was given a detailed verbal and written description of the study. After understanding the potential benefits and risks the subjects provided written informed consent. The study group consisted of 40 systemically healthy subjects (22 males and 18 females) falling in the age group of 21-63 years with mean age of 36.17± 10.72 years. However the values of the control group subjects (healthy subjects) were not included in the study. There was no change in the diet or any other habits during the study period.
Subjects participating in the study
| Group I | No of subjects-10 | Healtdy | clinical attachment level [CAL] 0-1mm | probing deptd (PD)≤ 2-3 mm |
| Group II | No of subjects-10 | Mild periodontitis | clinical attachment level (CAL) 1–2 mm | probing depth (PD) 3–4 mm |
| Group III | No of subjects-10 | Moderate periodontitis | CAL 3–4 mm) | (PD) 5-7 mm), |
| Group IV | No of subjects-10 | Severe periodontitis | CAL •5 mm | (PD) >7 mm |
Preparation and Blood Collection: After the appropriate medical and dental history concerning the absolute and relative contraindications was procured, a thorough dental and periodontal examination was carried out. A modified plaque index [14] and probing pocket depth (in mm) measured with a UNC 15 probe were recorded. Then 3mL of venous blood was collected from each subject, of which 1 mL was maintained in a complete blood count (CBC) kit and 2 mL was stored in EDTA-containing vials to be analysed for fibrinogen degradation products (FDP). The EDTA-containing vials were taken to the Blood Bank of SGT Medical College and centrifuged, and platelet-poor plasma (PPP) was prepared within 1 hour of collection of the sample. The PPP thus prepared was frozen and sent to a pathology laboratory within 4–6 hours for estimation of D-dimer concentration by VIDAS®. The CBC kit was immediately transferred to the laboratory of SGT Dental College and Hospital for cell counts.
VIDAS and D-Dimer: The VIDAS® Immunoassay Testing System combines an ELISA with a final fluorescent reading Enzyme Linked Fluorescent Assay (ELFA assay), ensuring excellent sensitivity and specificity and is the first D-dimer test cleared by the U.S. Food and Drug Administration for exclusion of Pulmonary Embolism (PE) and Deep Vein Thrombosis (DVT). It contains a single-dose, ready-to-use reagent strip system. The SPR® (solid phase receptacle) is coated with mouse anti Fibrin degradation products (anti-FbDP) monoclonal immunoglobulins. The foil of the first well is perforated to facilitate introduction of the sample. The last well is a cuvette in which the fluorometric reading is performed. At each stage of the reaction, the Solid Phase Receptacle (SPR) aspirates the reagents in and out, preventing inter-reagent or inter-sample contamination. All of the assay steps are performed automatically by the instrument. The conjugate enzyme catalyzes the hydrolysis of the substrate, 4-methyl-umbelliferyl phosphate, into a fluorescent product (4-methyl-methyl-umbelliferyl), the fluorescence of which is measured at 450 nm. The intensity of the fluorescence is proportional to the concentration of antigen in the sample. The results are automatically calculated in relation to a calibration curve and stored in memory, while a report is printed for the user.
The measurement range of the VIDAS® D-dimer Exclusion II starts at 45ng/mL Fibrinogen Equivalent Units (FEU) with an upper limit of 10,000 ng/mL. The linearity has been determined on plasma according to the recommendations of 21Clinical Laboratory and Standards Institute (CLSI®) document EP6-A. The measurement is linear throughout the range [15].
Statistical Analysis
Data was analysed using two-way analysis of variance (ANOVA) followed by the Student–Newman Keuls multiple comparison test. Pearson’s correlation coefficient of various indices and the concentrations of FDP were calculated for each group. Statistical analysis showing a confidence level above 95% (p<0.05) was considered significant. All analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The results were presented as mean±standard deviation.
Results
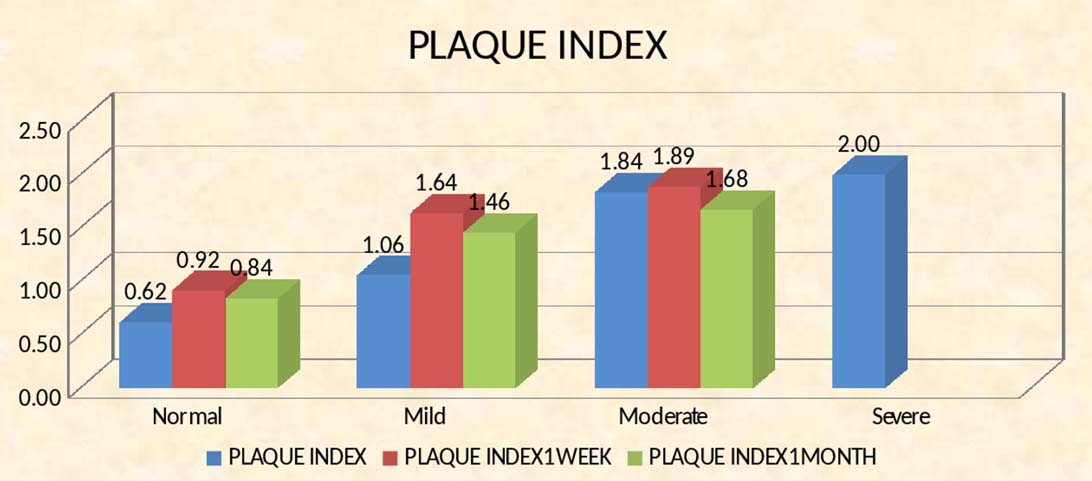
There was a significant improvement in the periodontal condition of each group after nonsurgical periodontal therapy, although many patients continued to have periodontitis [Table/Fig-2].
Plaque index depicting high scores with increasing severity of periodontitis
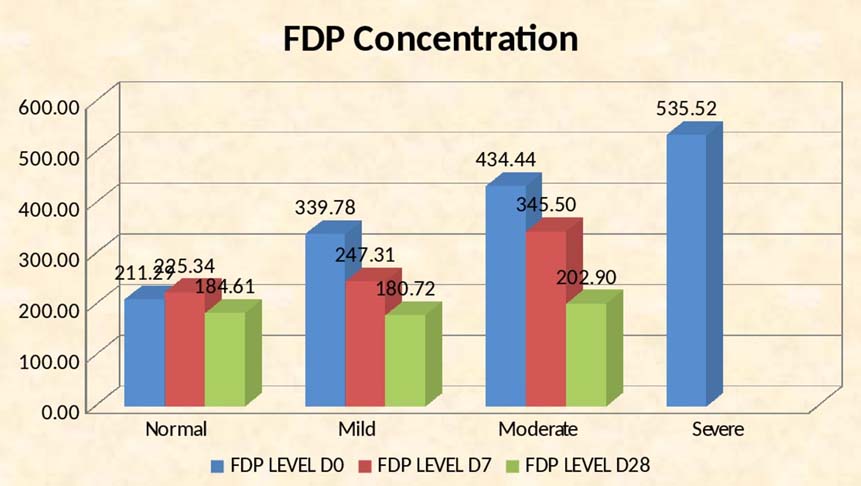
The FDP activity and periodontal parameters showed similar patterns of variation [Table/Fig-3], being highest in subjects with severe periodontitis and showing a striking reduction after periodontal therapy (535.52±44.71 vs. 202.90±31.57). In subjects with moderate periodontitis, the FDP concentration declined from 434.44±19.86 to 180.72±9.71 and in mild periodontitis from 339.78±37.39 to 184.61±8.42. Correlation of the FDP concentrations at baseline and on days 7 and 28 showed a reduction in the differences between the various grades of periodontitis (post-hoc test) [Table/Fig-4]. There was a significant improvement in the serum FDP concentration in all groups [Table/Fig-5].
Response of Concentrations of FDP to treatment according to degree of periodontitis (Mean {SD})
| NormalSubjects | MildPeriodontitis | ModeratePeriodontitis | SeverePeriodontitis |
|---|
| Baseline | 211.29(20.32) | 339.78(37.39) | 434.44(19.86) | 535.52(44.71) |
| 1week | | 225.34(15.74) | 247.31(33.80) | 345.50(71.97) |
| 4 weeks | | 184.61(8.42) | 180.72(9.71) | 202.90(31.57) |
Normal range D-dimer units110–250 ng/mL; fibrinogen equivalent units 0.22–050 mcg/mL
There was a significant improvement in the serum FDP concentration in all groups
Significance of differences in d-dimer concentrations with treatment according to degree of periodontitis
| p-Value at Baseline | p-Value at7 Days | p-Value at28 Days |
|---|
| Mild vs moderate | 0.004 | 1 | 1 |
| Moderate vs severe | 0.001 | 0.001 | 0.316 |
| Mild vs severe | 0 | 0.004 | 0.526 |
Discussion
Periodontitis is marked by gingival inflammation and loss of connective tissue and bone from around the roots of teeth [16], creating inflamed soft-tissue pockets between the gingival and the root surface. Periodontitis is a part of, not only of the systemic inflammatory response, but also of a significant disturbance of the endothelial and haemostatic system. Acute inflammation, whether from infection or other causes, could trigger acute vascular events, possibly by destabilizing the endothelium and coagulation system [17]. Lanz et al., demonstrated that Porphymonas gingivalis binds and degrades fibrinogen [8,9], confirming earlier findings that this common oral microorganism has fibrinogenolytic and fibrinolytic properties.
Fibrinogenolytic activity is the result of the cysteine proteinases gingipain-K and gingipain-R [18,19]. The activity of plasma gingipain-K is countered by albumin, high-molecular-weight kininogen, prekallikrein, and factor X, all of which are cleaved by proteinases of P. gingivalis [20–23]. Therefore, it seemed important to examine the fibrinogenolytic function of these enzymes directly in plasma. Plasminogen Activator Inhibitor-1 (PAI-1) is an important inhibitor of fibrinolysis, and D-dimer is released during the dissolution of a fibrin clot [24].
In this study, we examined periodontal inflammation as a risk factor for a procoagulant state and hence a common trigger of coagulation. We found that the concentration of FDP, including D-dimer, differs drastically with the severity of periodontal disease.
High D-dimer values are abnormal but do not indicate a specific disease state. For example, values may be increased as a result of clinical or subclinical disseminated intravascular coagulation/intravascular coagulation and fibrinolysis. Other conditions associated with increased activation of the procoagulant and fibrinolytic mechanisms are recent surgery, active or recent bleeding, haematomas, trauma or thromboembolism, pregnancy, liver disease, inflammation, malignancy, or a hypercoagulable (procoagulant) state [25].
Graziani F et al., examined the level of FDP 24 hours after surgical and non-surgical periodontal therapy. Their results showed that the levels of D-dimer increased tremendously 24 hour post non-surgical therapy (p<0.05) than surgical therapy. Hence they observed that systemic inflammation increased more after nonsurgical periodontal therapy as compared to surgical periodontal therapy. However, in the present study the D-dimer levels have been estimated at baseline, 7 days and 28 days with a significant reduction from the baseline [26].
Practical Implications: Periodontal disease is associated with serum Fibrinogen Degradation Products (FDP). It would be valuable to determine the sensitivity and specificity of FDP as biomarkers for periodontal disease activity and a screening tool.
Clinical Relevance
Scientific Rationale for the Study: We examined whether the plasma D-dimer concentration reflects the extent of chronic periodontitis and the beneficial effect of therapy.
Principal Findings: Patients with moderate and chronic periodontitis exhibited high concentrations of D-dimer (mean 134.98–535.52 mcg/mL), whereas subjects with mild or no periodontitis exhibited values of 339.78–211.29 mcg/mL. Concentrations were significantly reduced after therapy.
Limitations
FDP can be raised in other non pathological conditions like pregnancy and a few other pathological states such Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), surgery, trauma, infection, inflammation and cancer. The results of the study need to be corroborated with a larger number of subjects over a longer duration of time.
Conclusion
The present study supports the hypothesis that progression of periodontal disease is associated with various amounts of serum FDP and the plasma D-dimer concentration reflects the severity of periodontitis and the beneficial effect of therapy. The rise in FDP was reduced by nonsurgical periodontal therapy in our study. The future of this work lies in establishing the sensitivity and specificity of FDP as a biomarker for periodontal disease activity. It could also be a screening tool and help in predicting the onset of disease, perhaps being valuable as a prognostic test.
Normal range D-dimer units110–250 ng/mL; fibrinogen equivalent units 0.22–050 mcg/mL