Evaluation of Endothelial and Platelet Derived Microparticles in Patients with Acute Coronary Syndrome
Melvin George1, M.R. Ganesh2, Aruna Sridhar3, Amrita Jena4, Muthukumar Rajaram5, Elangovan Shanmugam6, V.E. Dhandapani7
1 Assistant Professor, Department of Cardiology, SRM Medical College Hospital & Research Centre, Tamil Nadu, India.
2 Assistant Professor, Interdisciplinary School of Indian System of Medicine, SRM University, Tamil Nadu, India.
3 Clinical Research Associate, Department of Cardiology, SRM Medical College Hospital & Research Centre, Tamil Nadu, India.
4 Clinical Research Associate, Department of Cardiology, SRM Medical College Hospital & Research Centre, Tamil Nadu, India.
5 Clinical Research Associate, Department of Cardiology, SRM Medical College Hospital & Research Centre, Tamil Nadu, India.
6 Professor, Department of Cardiology, SRM Medical College Hospital & Research Centre, Tamil Nadu, India.
7 HOD and Professor, Department of Cardiology, SRM Medical College Hospital & Research Centre, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Melvin George, Department of Cardiology, Cardiac Clinical Trials & Research, SRM University, SRM Nagar, Kattankulathur, Kancheepuram District, Tamil Nadu-603203, India.
E-mail: melvingeorge2003@gmail.com
Background
Microparticles (MP) are a nuclear fragments of membrane released by the damaged cell during stress. Elevated levels of MP have been found in patients with acute coronary syndrome (ACS) owing to the damage in the endothelium.
Aim
To determine if the levels of endothelial and platelet microparticles (EMP & PMP) in patients with ACS influenced the severity of the disease.
Materials and Methods
This was a prospective cohort study performed in 63 ACS patients (ST elevation myocardial infarction- STEMI-28, non ST elevation myocardial infarction -NSTEMI-35). After obtaining consent, blood samples were collected from the patients and processed by flow cytometry.
Results
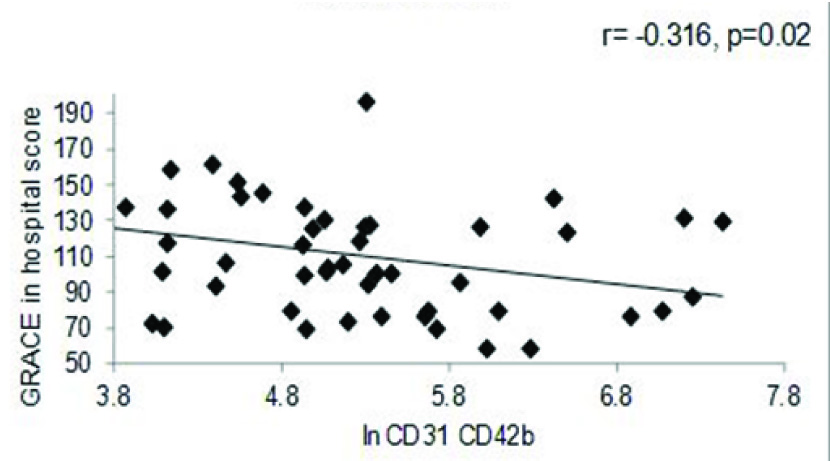
The NSTEMI group had higher levels of EMP {792.11(327.59-1661.49) vs 300.35 (176.3-550.46), p=0.001} and PMP {218.87(86.65-439.77) vs 114.45(50.34-196.75), p= 0.007} as compared to the STEMI group. However, it was found that the EMP (r=-0.438, p=0.001) and PMP (r= -0.316, p=0.024) negatively correlated with Global Registry of Acute Coronary Events score (GRACE in-hospital score) for the entire cohort.
Conclusion
The levels of microparticles are elevated in ACS patients and may reflect a protective effect in patients with acute coronary syndrome.
Endothelial microparticles, GRACE Score, Myocardial infarction, Platelet microparticles
Introduction
According to the World Health Organization (WHO) 7.4million deaths were attributed to ischemic heart diseases and it was on the top for WHO’s 10 leading causes of death [1]. Even among developing nations like India, Cardiovascular disease (CVD) is one of the top 5 causes of death. In the Indian subcontinent, more than 25% of deaths can be attributed to cardiovascular diseases [2]. It has been estimated that in the next 15 years, India would have the maximum number of patients with cardiovascular ailments [3]. Acute coronary syndrome (ACS) is characterized by occlusion of the coronary arteries and comprises of ST Elevation Myocardial Infarction(STEMI), Non ST Elevation Myocardial Infarction (NSTEMI), or Unstable Angina (UA). According to the American Heart Association, every year 2.5 million people are hospitalized for ACS out of which approximately 18% of women and 23% of men above the age of 40 years die within a year of being diagnosed as Myocardial Infarction(MI) [4]. MI is caused due to the formation and rupture of unstable atherosclerotic plaques. These plaques are formed by the adhesion and migration of lipids, and inflammatory cells to the inner layer/tunica intima of the arterial walls, chiefly aided by endothelial dysfunction. This unchecked inflammatory build-up of plaque not only narrows down the arterial lumen and occludes blood flow but also leads to MI in case of plaque rupture and thrombosis [5].
Endothelial dysfunction can lead to loss of endothelial mono layer’s anticoagulant, antiplatelet and fibrinolytic properties. During endothelial dysfunction, considerable cell damage occurs due to apoptosis. A nuclear fragment of membrane is released by the stressed/damaged cell which are termed as “microparticles”. It comprises of cytoplasmic material from their cell of origin and cell surface proteins. These MPs range from 0.1-1.0μm and can be traced back to their origin using the surface proteins. They contain phospholipids and membrane from the cell they originated, enabling differentiation between them partly. Based on the surface adhesion molecules they can be classified into: platelet microparticles or PMP (CD41a, CD42b, CD62P), endothelial microparticles or EMP (CD144, CD62E, or CD31), leukocyte microparticles (CD45, CD4, CD8, CD14) and erythrocyte microparticles (CD235a) [6]. They perform a plethora of activities such as facilitating intercellular interactions, inducing cell signaling, and transferring receptors between different cell types [7]. Endothelial repair is brought about by Endothelial Progenitor Cells (EPC). This is influenced by the levels of EMPs and PMPs along with Circulating Endothelial Cells (CEC) as they impair the activity of EPC.
Recent evidence suggests that elevated levels of microparticles are found in ACS patients owing to the damage in the endothelium [8]. EMP (CD31) and PMP (CD31 CD42b) are known to be elevated in patients with ACS. In a study done by Sinning et al., it was seen that elevated levels of CD31+/Annexin V + were associated with a higher risk in Major Adverse Cardiac Events (MACE) in patients with stable Coronary Artery Disease (CAD) [9]. According to a study done by Nozaki et al., it can be said that high levels of circulating MPs can be used as a predictor for cardiovascular death in patients with ACS [10]. Piccin et al., have reported elevated levels of PMP (CD42b) in patients with ACS [11]. In a study done by Zee et al., they found elevated levels of PMP in both STEMI and NSTEMI [12]. Similar findings were observed in a study done by Biasucci et al., [13].
Estimation of the levels of these MPs would help assess the extent of damage and the degree of endothelial repair that has occurred. The objective of this study was to compare the levels of EMPs and platelet PMPs between the ST-Elevation Acute Coronary Syndrome or ST-ACS and Non–ST-Elevation Acute Coronary Syndrome or non ST-ACS (includes NSTEMI and UA) groups and to see if these microparticles correlated with disease severity.
Materials and Methods
The study protocol was approved by the SRM Hospital Ethics Committee, Kattankulathur, TN, India. Consecutive patients with a diagnosis of ACS as confirmed by appropriate changes in ECG and cardiac biomarkers, above 18 years of age, either gender, and willingness to give written informed consent were included in the study, between January 2014 to April 2014 in the Department of Cardiology. We excluded patients presenting with ACS symptoms 48 hours after arrival to the hospital. Blood samples were collected from each patient, and analysed for EMP and PMP using flow cytometry by following the protocol given below. In addition to patients, we also measured EMP and PMP in 6 healthy volunteers for comparison. Healthy volunteers were recruited from the hospital staff who were willing to participate.
Quantification of MPs was carried out on blood samples obtained from subjects as per previously reported method with modifications to the protocol [14]. Briefly, 2ml blood samples were drawn into Ethylene Diamine Tetra Acetic Acid (EDTA) containing vacutainers from the study subjects. The blood sample was collected within 24 hours of hospital admission. The blood sample obtained was immediately centrifuged for 10min at 1500g to obtain Platelet Rich Plasma (PRP). PRP was further centrifuged for 10min at 13000g to obtain Platelet Poor Plasma (PPP). PPP was labeled with appropriate fluorophores and used for further downstream Fluorescence Activated Cell Sorting (FACS) analysis. A 30 μl of the PPP was incubated with 4μl of Platelet Endothelial Cell Adhesion Molecule (PECAM-1) /anti-CD-31-PE and anti-CD-42b- Fluorescein isothiocyanate /anti-CD-42b-FITC each. The sample was further diluted with 750 μl of Phosphate Buffered Saline (PBS). A known concentration of 1 micron and 2 Micron Microsphere (MS) were added to each of the samples to serve as internal standards as well as to aid in calculating the absolute MP numbers. Samples were then analysed using BD FACS caliber and the data analysed using Cellquest software. MPs smaller than 1 μm and double positive for both CD31 and CD42 were defined as PMPs while MPs of size < 1μm but only positive for CD31 were defined as EMPs. Absolute MP (Amp) numbers for individual samples were calculated using the formula and used for comparison between samples.
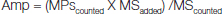
To assess the severity of the angiographic lesions of the study subjects, a modified Gensini score for each patient was calculated according to the method mentioned in the study done by Vlietstra et al., [15]. The GRACE score and TIMI risk score were also calculated for all the patients to determine the risk levels with respect to MPs. These risk scores were calculated using web based risk score calculators [16–18]. All patients were treated as per standard hospital treatment guidelines.
Statistical Analysis
Data were expressed as mean±SD or median with the inter-quartile range. The patients were classified into two groups based on ST changes in ECG as ST-ACS and non-ST ACS. The baseline characteristics of the patients in these two groups were compared using student’s t-test or Pearson’s chi-square test. Q-Q plot was used to assess normality of data. Mann-Whitney Test was done to compare the MP levels between the two groups. MP number was logarithmically transformed and Pearson correlation was done to assess its association with risk factors such as age, creatinine, left ventricular ejection fraction (LVEF) and GRACE score. The Gensini score was compared with the levels of microparticles to assess the severity of the lesions with respect to the MPs. Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS v17.0) software.
Results
A total of 63 ACS patients participated in the current study and were further divided into 2 subgroups based on the type of ACS; ST-ACS (n=28) and non-ST ACS (n=35). [Table/Fig-1] shows the baseline data for the 2 ACS sub groups. The most striking difference between the 2 sub-groups was the lower mean LVEF in ST-ACS {46.63% vs. 55.73%, (p=0.001)}. The data also indicated an increased incidence of ST-ACS in males (p=0.05), smokers (p=0.05) and patients with higher blood sugar levels (p=0.01). There was no significant difference between the groups in other characteristics such as age, body mass index (BMI), diabetes mellitus (DM), hypertension (HTN), lipid profile, renal dysfunction and history of ACS. The median EMP for the ACS patients was found to be 440.87 (220.08-1264.92) while that in the healthy volunteers was found to be 243.51 (176.18-485.64). The median PMP levels in ACS patients of our study was found to be 268.49 (60.94-287.11) while that in the healthy volunteers was found to be 63.34 (34.67-93.91).
Baseline characteristics of study patients
| Characteristics | Total(n=63) | ST ACS(n=28) | Non –STACS (n=35) | p-value |
|---|
| Age in years | 52.52 ± 10.83 | 52.61 ± 12.5 | 52.46 ± 9.48 | 0.86 |
| Gender | 65.7(46) | 85.7(24) | 62.9(22) | 0.04 |
| BMI | 26.14 ± 4.2 | 25.2 ± 3.66 | 26.86 ± 4.49 | 0.15 |
| DM | 47.1(33) | 50(14) | 54.3(19) | 0.73 |
| HT | 48.6(34) | 57.1(16) | 51.4(18) | 0.65 |
| Dyslipidemia | 45.7(32) | 64.3(18) | 40(14) | 0.08 |
| Smoking | 21.4(15) | 35.7(10) | 14.3(5) | 0.05 |
| HACS | 7.1(5) | 10.7(3) | 14.3(5) | 0.46 |
| FACS | 17.1(12) | 10.7(3) | 25.7(9) | 0.13 |
| TC | 189.6 ± 42.49 | 196.61 ± 40.89 | 182.33 ± 43.66 | 0.26 |
| HDL | 36.18 ± 8.32 | 36.5 ± 9.1 | 35.85 ± 7.59 | 0.86 |
| VLDL | 33.46 ± 41.01 | 25.16 ± 9.99 | 41.76 ± 56.48 | 0.15 |
| TGL | 178.28 ± 139.81 | 177.21 ± 138.11 | 179.42 ± 144.34 | 0.89 |
| LDL | 126.05 ± 38.14 | 131 ± 37.5 | 120.93 ± 38.82 | 0.6 |
| RBS | 163.13 ± 70 | 196.35 ± 79.06 | 142.38 ± 55.44 | 0.01 |
| Urea | 26.08 ± 10.31 | 27.25 ± 12.06 | 25.14 ± 8.73 | 0.78 |
| Creatinine | 1 ± 0.3 | 1.04 ± 0.3 | 0.97 ± 0.3 | 0.18 |
| LVEF | 51.27 ± 11.52 | 46.63 ± 11.09 | 55.73 ± 10.16 | 0.001 |
BMI- Body mass index; DM-Diabetes mellitus; HT-Hypertension; HACS-History of Acute Coronary Syndrome; FACS- Family history of Acute Coronary Syndrome; TC-Total cholesterol; HDL-High density lipoprotein; VLDL- Very low density lipoprotein; TGL-Triglycerides; LDL-Low density lipoprotein; RBS-Random blood sugar;n LVEF- Left Ventricular Ejection Fraction.
EMP and PMP levels between ST-ACS and non-ST ACS patients were compared [Table/Fig-2a]. There was a significantly increased level of EMP found in the non-ST ACS group compared to the ST-ACS group {792.11(327.59-1661.49) vs 300.35 (176.3-550.46), p=0.001}. Also, between the 2 sub- groups, the level of PMP in non-ST ACS was found to be significantly higher as compared to the ST-ACS group {218.87(86.65-439.77) vs 114.45(50.34-196.75), p= 0.007}. A sample output of the microparticle assay using BD FACS is depicted [Table/Fig-2b,c].
Level of microparticles in ST-ACS and non- ST-ACS
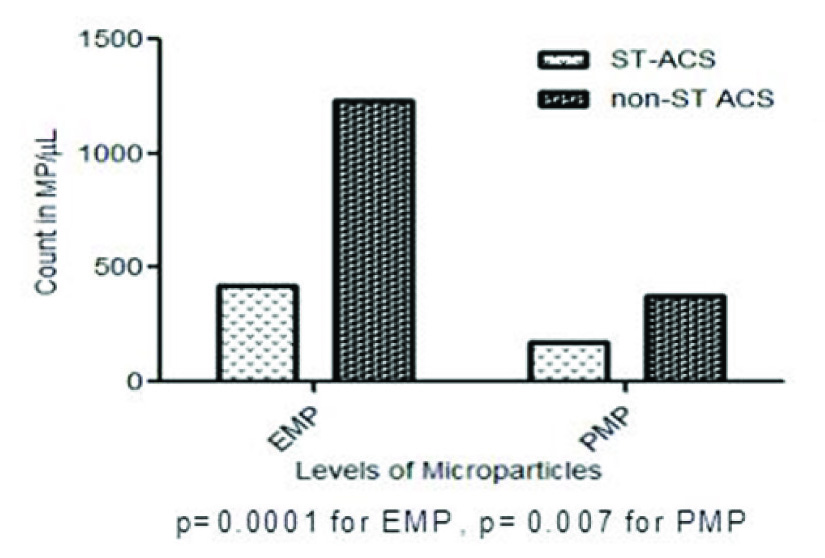
(b) The representative dot plots of ST-ACS subjects show, increased EMP levels (upper left quadrant) and PMP levels (upper right quadrant). (c) The representative dot plots of non-ST-ACS subjects show, increased EMP levels (upper left quadrant) and PMP levels (upper right quadrant)
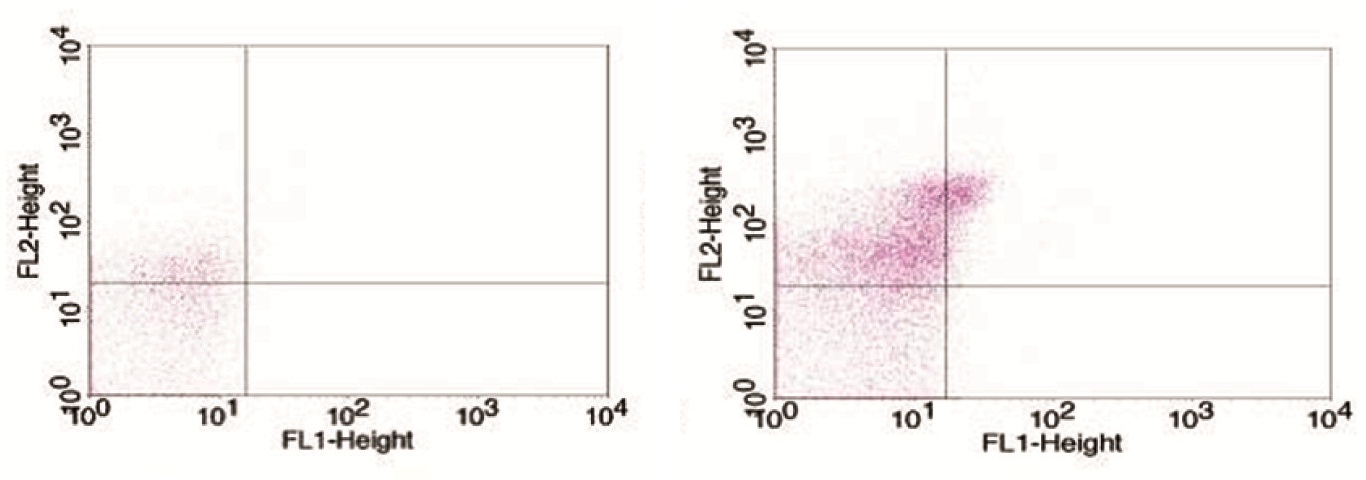
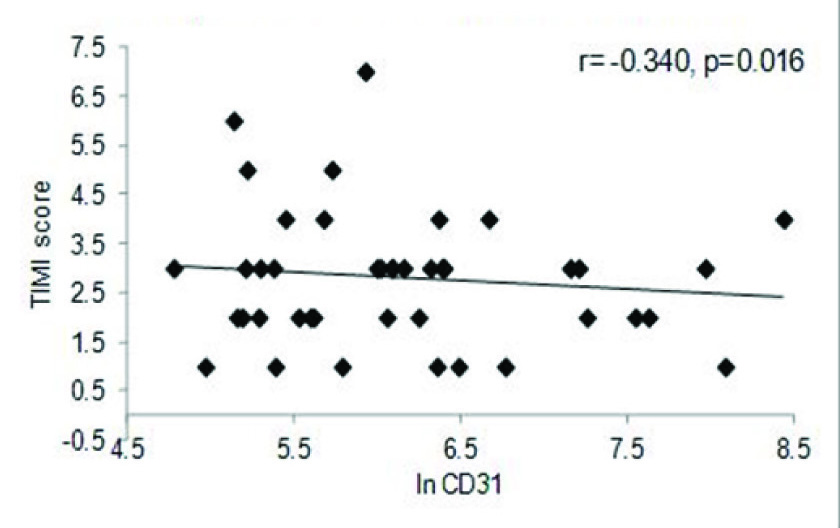
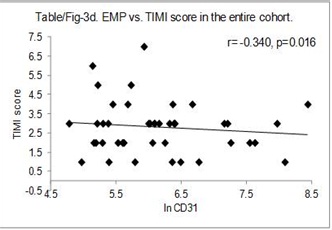
The correlation between GRACE – in hospital score and MP levels were studied for the entire cohort. The data indicated a significant negative correlation between both EMP (r=-0.438, p=0.001) [Table/Fig-3a] and PMP levels (r= -0.316, p=0.024) [Table/Fig-3b] for the entire cohort. Similarly, negative correlation was also observed between EMP and GRACE score for 6 months (r= -0.363, p=0.009) [Table/Fig-3c] as well as, negative correlation between EMP and TIMI score (r= -.340, p=0.016) [Table/Fig-3d] for the entire cohort.
EMP vs. GRACE in hospital score for the entire cohort
PMP vs. GRACE score in hospital for the entire cohort
EMP vs. GRACE score (6-months) in the entire cohort
EMP vs. GRACE score in the entire cohort
A strong positive correlation was also observed between EF and In of EMP (r=0.36, p= 0.006), as well as between PMP and EF (r=0.37, p=0.007) in the entire cohort [Table/Fig-4a,b]. A similar trend was seen with respect to BMI (r=0.30, p= 0.01) and triglycerides (r=0.39, p=0.04) (data not shown). The correlation between EF and MP levels was further reflected in the MI cohort, where once again both in values of EMP (r=0.72, p=0.001) and PMP levels (r=0.61, p= 0.001) showed significant positive correlation to the EF percentage [Table/Fig-4c,d]. Comparison between values of both the PMP and EMP show a significant positive correlation for both in the entire cohort (r=0.88, p=0.001) as well as the MI group (r=0.90, p=0.001) [Table/Fig-5a,b].
EMP vs. Ejection Fraction in the entire cohort
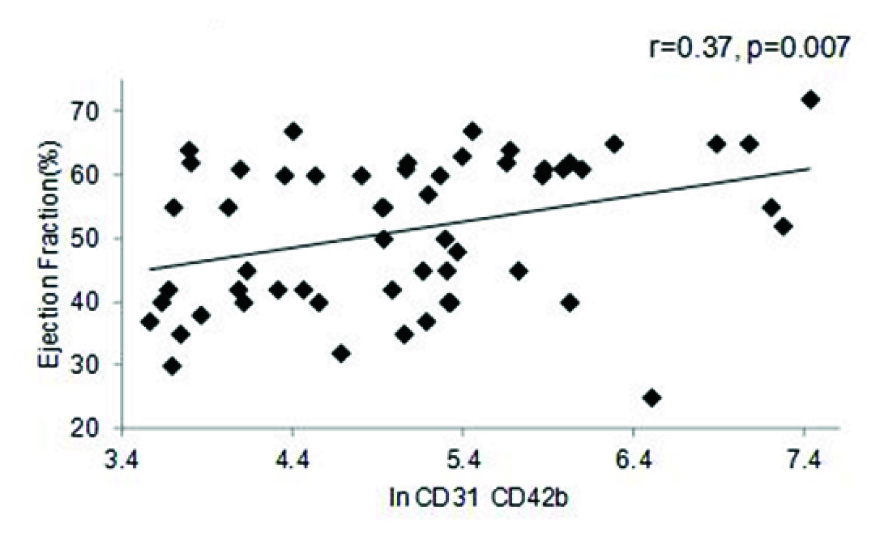
PMP vs. Ejection Fraction in the entire cohort
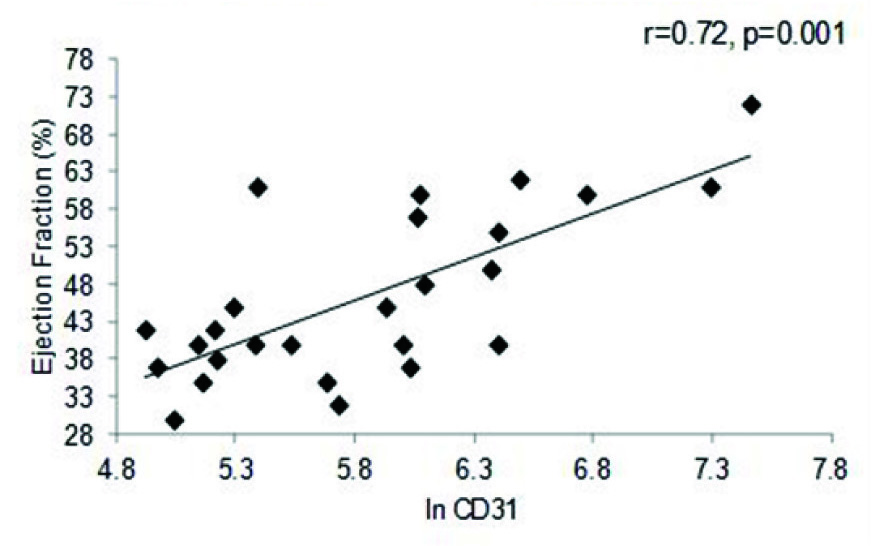
PMP vs. Ejection Fraction in the MI cohort
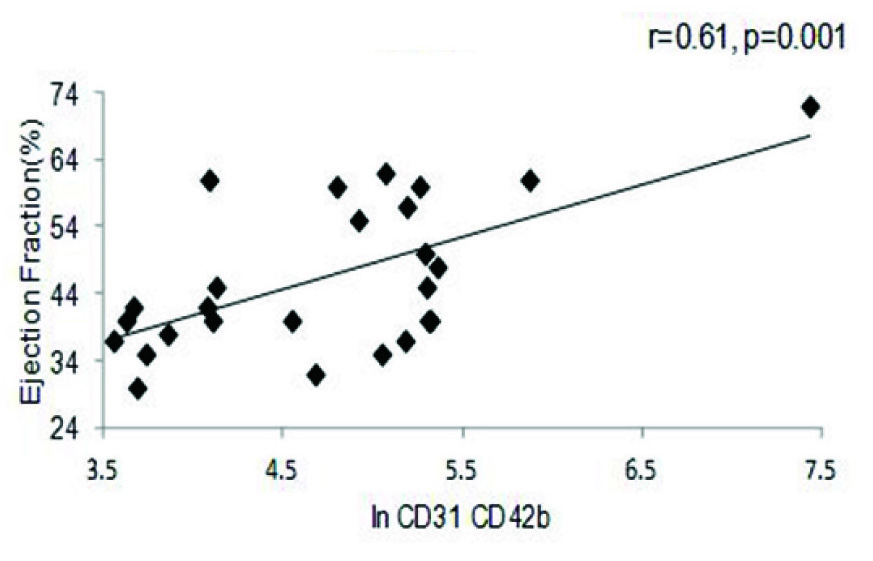
EMP vs. Ejection Fraction in the MI cohort
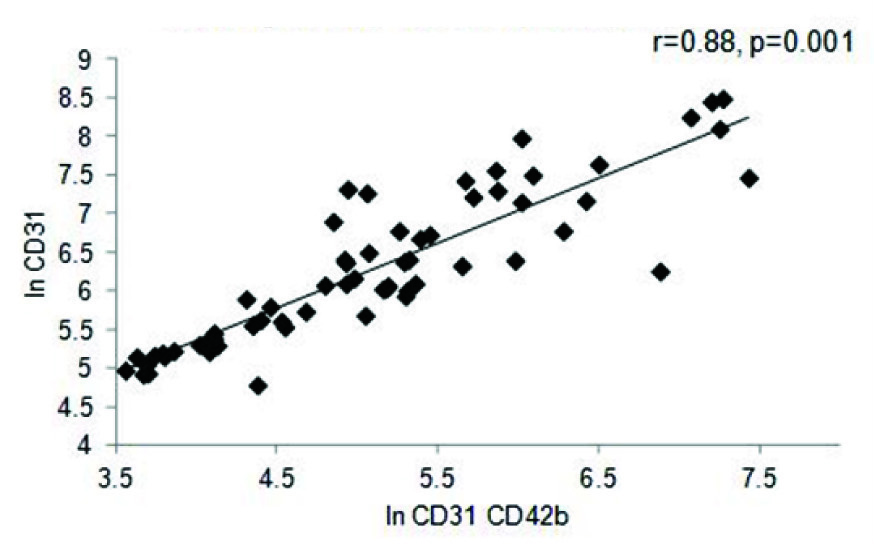
PMP vs. EMP in the entire cohort
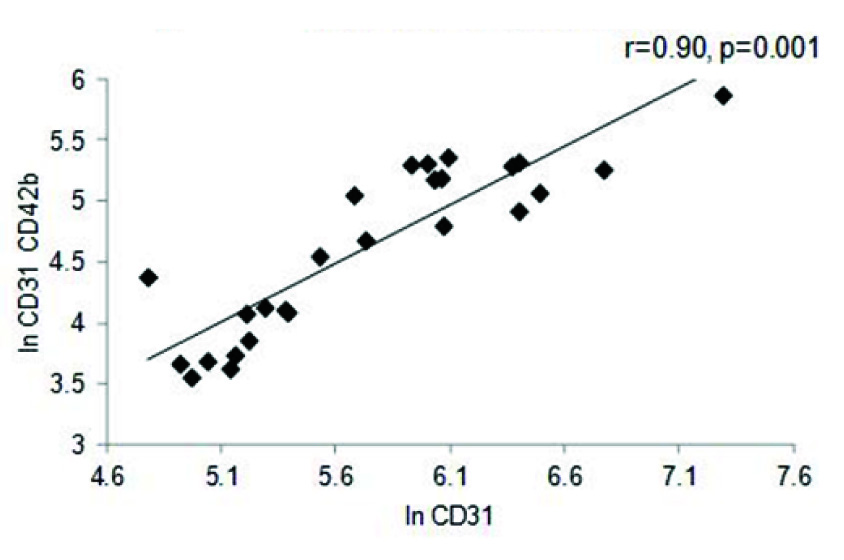
EMP vs. PMP in the MI cohort

Discussion
To the best of our knowledge this is the first study done in Indian patients that has estimated the levels of EMPs and PMPs in patients with ACS. In our study we compared the levels of EMP and PMP between ST-ACS and non-ST ACS. The EMP level for the non-ST ACS cohort was found to be higher as compared to the ST-ACS cohort. The same trend was followed by PMPs as well. In contrast the study by Biasucci et al., in Italian population did not show any difference between STEMI and NSTEMI. However, they observed that patients with ACS had higher MP than In line number 15 SA is mentioned for the first time so expansion for SA i.e. stable angina should be mentioned there and not directly in line number 17 where it is mentioned for the second time for better understanding. In another study done by Cui et al., they found significant differences in the levels of EMP amongst stable angina (SA), myocardial infarction (MI), UA and control. The same trend was followed by PMPs as well. However, they did not find any difference between MI and UA or between SA and control for both EMP and PMP [19]. In a study done by Mallat et al., with ACS patients, they did not find any difference between MI and UA patients as far as EMP levels were concerned. However the levels of procoagulant MPs were higher in the ACS cohort as compared to controls and stable angina [8]. Based on these earlier reports, it appears that these MPs are released in the setting of ACS and come down to normal levels once the disease is stabilized. Although other studies did not show any difference between ST-ACS and non- ST-ACS, the low EMP/PMP in ST-ACS group could argue for its protective role in coronary artery disease. It is not known if deficiency of microparticles could contribute to the aetiopathology of ST-ACS.
The GRACE score is a reliable tool to stratify patients with acute coronary syndrome and predict in-hospital and 6-month mortality in these patients. In our study we found that GRACE score (in-hospital) correlated inversely with levels of EMP and PMP. The same was observed for GRACE score (6months) and TIMI score with respect to EMPs. This could perhaps reflect the protective role offered by MPs in coronary artery disease since the level of MPs is maximal in individuals with low GRACE score. This could imply that, the levels of MPs are inversely proportional to the GRACE and TIMI score, again emphasizing the protagonistic role of the MPs. MPs help in intracellular communication and protect the cell from stress. They are rich in phospholipids, chemotherapeutic substances and caspase 3. This caspase 3 containing MPs are periodically released out, but when this flushing out is inhibited, it leads to abnormally elevated levels of caspase 3 [20]. In such a state, the MPs have an automechanism to prevent accumulation of caspase 3, reiterating the protective role of MPs.
In our study, we found that EMP as well as PMP showed direct correlation with ejection fraction. This could suggest that EMPs and PMPs are abundant in patients with preserved EF, its levels diminish as the LV function worsens. It is not known as to why MPs decrease in patients with LV dysfunction. In a study done by Kuliczkowski et al., they found elevated levels of EPCs and EMP in MI patients who had preserved EF post Percutaneous Transluminal Coronary Angioplasty/PTCA [21]. All the sample collection in our study was done prior to PCI. In a study done by Bal et al., they found that PMPs did not show any correlation with EF [22].
Though microparticles can be used as an effective tool for detection of endothelial dysfunction, there are considerable challenges involved in their estimation. They have a diverse size range and it is hard to develop an assay which would easily detect them with high sensitivity and specificity. They are also eliminated from the system rapidly, making their detection even more difficult.
Limitation
The main limitation of our study was the small sample size. The small sample size precluded us from obtaining sufficient number of cardiovascular events to correlate MPs concentration with cardiovascular outcomes. As the study was cross sectional in nature, the finding of a protective role of micro particles should only be considered hypothesis generating and requires rigorous scrutiny using prospective study designs. Undoubtedly these findings need to be replicated in larger populations to understand the exact pathogenic role of microparticles in acute coronary syndrome.
Conclusion
Microparticles are elevated in patients with ACS and they are found to correlate inversely with the GRACE and TIMI score and this could suggest a protective role of microparticles in coronary artery disease.
BMI- Body mass index; DM-Diabetes mellitus; HT-Hypertension; HACS-History of Acute Coronary Syndrome; FACS- Family history of Acute Coronary Syndrome; TC-Total cholesterol; HDL-High density lipoprotein; VLDL- Very low density lipoprotein; TGL-Triglycerides; LDL-Low density lipoprotein; RBS-Random blood sugar;n LVEF- Left Ventricular Ejection Fraction.
[1]. WHO | The top 10 causes of death [Internet]. WHO. [cited 2015 Jul 14]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/ [Google Scholar]
[2]. Gupta S, Bhise M, Gaurav K, Gudapati R, Emerging risk factors for cardiovascular diseases: Indian contextIndian J Endocrinol Metab 2013 17(5):806 [Google Scholar]
[3]. Gupta R, Joshi P, Mohan V, Reddy KS, Yusuf S, Epidemiology and causation of coronary heart disease and stroke in IndiaHeart Br Card Soc 2008 94(1):16-26. [Google Scholar]
[4]. WHO | The Atlas of Heart Disease and Stroke [Internet]. WHO. [cited 2014 Oct 30]. Available from: http://www.who.int/cardiovascular_diseases/resources/atlas/en/ [Google Scholar]
[5]. Heart attack: MedlinePlus Medical Encyclopedia [Internet]. [cited 2014 Dec 11]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/000195.htm [Google Scholar]
[6]. Burger D, Touyz RM, Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cellsJ Am Soc Hypertens 2012 6(2):85-99. [Google Scholar]
[7]. Fan Y, Wang L, Li Y, Yin Z, Xu Z, Wang C, Quantification of endothelial microparticles on modified cytometric bead assay and prognosis in chest pain patientsCirc J 2014 :206-14. [Google Scholar]
[8]. Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromesCirculation 2000 101(8):841-43. [Google Scholar]
[9]. Sinning JM, Losch J, Walenta K, Böhm M, Nickenig G, Werner N, Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomesEur Heart J 2011 32(16):2034-41. [Google Scholar]
[10]. Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y, Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart diseaseJ Am Coll Cardiol 2009 54(7):601-08. [Google Scholar]
[11]. Piccin A, Murphy WG, Smith OP, Circulating microparticles: pathophysiology and clinical implicationsBlood Rev 2007 21(3):157-71. [Google Scholar]
[12]. Van der Zee PM, Biró E, Ko Y, de Winter RJ, Hack CE, Sturk A, P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarctionClin Chem 2006 52(4):657-64. [Google Scholar]
[13]. Biasucci LM, Porto I, Di Vito L, De Maria GL, Leone AM, Tinelli G, Differences in microparticle release in patients with acute coronary syndrome and stable anginaCirc J Off J Jpn Circ Soc 2012 76(9):2174-82. [Google Scholar]
[14]. Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, Endothelial microparticles correlate with erectile dysfunction in diabetic menInt J Impot Res 2006 19(2):161-66. [Google Scholar]
[15]. Vlietstra RE, Kronmal RA, Frye RL, Seth AK, Tristani FE, Killip T, Factors affecting the extent and severity of coronary artery disease in patients enrolled in the coronary artery surgery studyArterioscler Thromb Vasc Biol 1982 2(3):208-15. [Google Scholar]
[16]. GRACE ACS Risk Model [Internet]. [cited 2014 Dec 12]. Available from http://www.outcomes-umassmed.org/grace/acs_risk/acs_risk_content.html [Google Scholar]
[17]. TIMI Risk Score for STEMI [Internet]. MDCalc. [cited 2014 Dec 11]. Available from: http://www.mdcalc.com/timi-risk-score-for-stemi/ [Google Scholar]
[18]. TIMI Risk Score for UA/NSTEMI [Internet]. MDCalc. [cited 2014 Dec 11]. Available from: http://www.mdcalc.com/timi-risk-score-for-uanstemi/ [Google Scholar]
[19]. Cui Y, Zheng L, Jiang M, Jia R, Zhang X, Quan Q, Circulating microparticles in patients with coronary heart disease and its correlation with interleukin-6 and C-reactive proteinMol Biol Rep 2013 40(11):6437-42. [Google Scholar]
[20]. Tushuizen ME, Diamant M, Sturk A, Nieuwland R, Cell-Derived Microparticles in the Pathogenesis of Cardiovascular Disease Friend or Foe?Arterioscler Thromb Vasc Biol 2011 31(1):4-9. [Google Scholar]
[21]. Kuliczkowski W, Derzhko R, Prajs I, Podolak-Dawidziak M, Serebruany VL, Endothelial progenitor cells and left ventricle function in patients with acute myocardial infarction: potential therapeutic considertionsAm J Ther 2012 19(1):44-50. [Google Scholar]
[22]. Bal L, Ederhy S, Di Angelantonio E, Toti F, Zobairi F, Dufaitre G, Factors influencing the level of circulating procoagulant microparticles in acute pulmonary embolismArch Cardiovasc Dis 2010 103(6-7):394-403. [Google Scholar]