Cancer is a major cause of death and disability across the world. According to the International Agency for Research on Cancer GLOBOCAN project, 2012, the cancer burden in India will double in the next 20 years i.e. by the year 2035, there will be more than 1.7 million new cases [1]. Also, the absolute number of cancer deaths will increase simultaneously in the corresponding period [1,2]. In India, the epidemiology of cancer is complex. The lack of a national cancer registry, high mortality among patients less than 70 years, local factors like tobacco use, smoking, poor-hygienic conditions, infections, poverty and limited access to treatment for the patients, make Indian scenario distinct from the rest of the world. This is further compounded by the poor awareness among the patients leading to less frequent screening and late reporting to the healthcare professional during the advanced stage of the disease [3].
Further, most cancer patients in India reel under the pressure of the astronomical cost of treatment [4]. Generally, the primary treatment options include surgery, chemotherapy, radiotherapy and palliative care. The choice of treatment depends upon the type and location of tumour, spread of the disease at the time of diagnosis and general health of the patient. Depending upon the stage of the cancer at the time of diagnosis, the aim of the treatment can vary from being curative to palliative. The overall cost including both the diagnosis and long term treatment increase the cost by many magnitudes. One major chunk of this costly treatment is the expense on anti-cancer drugs. These exorbitantly priced anti-cancer drugs are crucial in the management protocol [4,5].
Apart from the cost of the treatment, the patients generally experience effects of the disease itself and/or side-effects of the cancer treatment. The combined effect of the disease and toxic drugs impair the quality of life of the patients and may make it difficult for them to undergo the treatment. Another issue, i.e. development of resistance of the cancerous cells to the drug therapy is of considerable importance for both the patient and the treating physician. The tumour may become resistant to chemotherapy after long-term administration, leading to poor response with the chemotherapeutic agents.
As cancer care involves a burden on the patients and society both, different options have been suggested to decrease the cost of cancer chemotherapy but simultaneously improve the outcome [4,6]. Inclusion of phytomedicine into the conventional therapy is one of the proposed methods. Phytomedicine, is herbal-based traditional medical practice that uses various plant materials in modalities considered both preventive and therapeutic. Clinical studies evaluating the efficacy of herbal drugs among cancer patients have shown variable contradictory results [7,8].
The Health Technology Assessment on Phytomedicine includes gathering and analysing evidences on clinical efficacy, safety, cost-effectiveness, feasibility and health system integration possibilities. Our aim was to know whether addition of herbal products can be another viable option to have cost-effective chemotherapy.
The objective of this review was: a) to understand the role of phytomedicine in treatment of cancer, b) to assess clinical effectiveness of phytomedicine, c) to assess cost effectiveness of phytomedicine and d) to assess mortality rate and quality of life.
Materials and Methods
Types of studies: Randomized controlled trials, observational analytical studies, case control and cohort studies were included. The experimental arm patients must have taken phytomedicine (it also includes studies of Chinese herbal medicine) and the control arm must have been administered conventional therapy for management of cancer.
Type of patients
All cancer (e.g. breast, prostate, lung, gastric, pancreas, osteosarcoma) patients who had been administered herbal products (phytomedicine) for treatment.
Search methods for identification of studies
Two independent reviewers independently searched the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews, MEDLINE, PubMed Science Direct, SCOPUS, EMBASE, LANCET, and Google Scholar for relevant studies published since 1987 till 2nd November 2014. The reference lists of key review articles were also searched for additional references. We also conducted specific searches for primary studies published more recently than the included systematic reviews and meta-analyses. The key words mainly used for article search were: phytomedicine and cancer, comparative study of phytomedicine and chemotherapy, phytomedicine and other treatment of cancer, herbal medicine and cancer.
The inclusion criteria for the studies were: a) intervention arm taking phytomedicine and the other comparator arm taking conventional therapy, b) outcome of the study must include mortality and effect on quality of life. We limited our search to studies published in the English language.
Selection of studies
Two authors independently screened the literature search results and obtained the full reports of all potentially relevant trials. We resolved any disagreements through discussion with a third author.
Data extraction and management
Two authors independently extracted data using a specifically developed data extraction forms. We resolved any disagreements through discussion with all of the review authors. We contacted the corresponding publication authors in all the cases where clinical data was not clear or there was missing data. All analyses were conducted on RevMan [9]. We calculated risk ratios and mean difference through a forest plot and assessed the extent of bias by the generation of the risk of bias graph to measure the same across studies and the risk of bias summary for assessing the bias for every individual study. Forest plot, which is a graphic representation of the meta-analysis, is based on the number of events in terms of mortality, effect of intervention, adverse events of the corresponding intervention and control group data. A study flow diagram was generated to represent the study selection and screening process. We assessed for inclusion of all potential studies we identified as a result of the search strategy. The studies which didn’t fall in our inclusion criteria were excluded. Two reviewers independently extracted data on the number of randomized participants, intervention and its components, outcomes generated and the number of events based on the intervention and control groups. The risk of bias was assessed according to the parameters specified by the International Cochrane Collaboration.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias of each trial using the Cochrane risk of bias form [10]. We resolved any disagreements by discussion between review authors. Six components were assessed: generation of the randomization sequence, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting and other biases (such as the trial stopped early). We categorized our judgments as either ’low’, ’high’, or ’unclear’ risk of bias, and described our reasons for doing so.
Statistical Analysis
We calculated results using risk ratios for dichotomous data and mean difference through continuous data, all were analysed in RevMan 5.3. Funnel plots were not considered as the total number of included studies was less than 10 in number for a particular outcome.
Results
Description of studies
[Table/Fig-1] includes the characteristics of included studies [7,8,11–22]
Study characteristics [7,8,11–22]
| S.No | Study. | Population | Age group | Intervention(Phytomedicine) | Control | Outcome |
|---|
| 1 | Biswal 2013 | Breast cancer | 51 years (range = 36-70) and 50.5 years (range = 32-71) | chemotherapy with oral Withania somnifera(n=100) | Chemotherapy alone (n=100) | Survival rate |
| 2 | Troger 2012 | Breast cancer | Intervention group 49.0 ± 7.8Control group 51.8 ± 7.8 | chemotherapy with Viscum album (n=28) | Chemotherapy alone (n=28) | Survival rate |
| 3 | Wei 2011 | Stage III nasopharyngeal cancer | | Chinese traditional medicine (Yanshu injection) in addition to intensity modulated radiation therapy (IMRT) and concomitant chemotherapy(n=30) | Intensity modulated radiation therapy (IMRT) and concomitant chemotherapy(n=30) | Survival rate |
| 4 | Zou 2003 | Non-small cell lung cancer | | Astragalus injection (AI) combined with chemotherapy(n=30) | Chemotherapy alone(n=30) | Survival rate |
| 5 | Kucuk 2001 | Prostate cancer | Intervention group 61 (51–71)Control group 61 (53–70)- | Radical prostatectomy and lycopene(n=15) | Radical prostatectomy (n=11) | PSA level |
| 6 | Kumar 2008 | Prostate cancer | Intervention group 60.94(+/−7.05)Control group 60.30(+/−6.54) | radical prostatectomy and lycopene(n=10) | Radical prostatectomy(n=11) | PSA level |
| 7 | Reeves 2013 | Prostate cancer | Intervention group62±12Control group 62±7 | Isoflavone followed by prostatectomy(n=42) | Placebo capsules followed by Prostatectomy(n=44) | Levels ofPSA ng/mL,Total Testosterone, ng/dL,Free Testosterone, pg/mL,Total Estrogen, pg/mL,Estradiol, ng/mL,Total Cholesterol, mg/dL |
| 8 | Breemen 2011 | Prostate cancer | 63.4±8.0-Intervention group69.2±7.8-Control group | Lycopene(n=26) | Placebo(n=21) | Biomarker-Plasma lycopene,(mmol/L),Prostate tissue lycopene(pmol/mg tissue),Prostate tissue 8-oxo-dG/106 dG,Plasma malondialdehyde(mmol/L) |
| 9 | Beuth 2008 | Breast cancer | Intervention group55.11±9.61Control group 54.63±10.16 | Conventional therapies and sME HELIXOR(n=167) | Conventional therapies(n=513) | Adverse drug reaction, relapse, metastases, operations. |
| 10 | Matthes 2008 | Pancreatic cancer | | conventional therapy and fermented mistletoe extract Iscador® (ISC) (n=201) | Conventional therapies(n=195) | Adverse drug reaction |
| 11 | Piao 2004 | Breast, ovarian andnon-small cell lung cancer | Intervention group52.6±9.4Control group 51.7±10.1 | Conventional therapies and sME HELIXOR (n=115) | Conventional therapies and immunomodulating phytopharmaconLentinan(n=109) | Adverse event (quality of life) |
| 12 | Troger 2014 | Breast cancer | Intervention group50.4 ± 6.9Control group 50.8 ± 8.0 | Conventional therapies and HELIXOR A (n=29) | Conventional therapies(n=30) | Adverse event (quality of life) |
| 13 | Longhi 2014 | Osteosarcoma | Intervention group28 (18–48)Control group 39 (11–66) | Conventional therapies and Viscum (n=9) | Conventional therapies and etoposide-topoisomerase II inhibitor(n=11) | Adverse event (quality of life) |
| 14 | Kim 2012 | Gastric Cancer | Intervention group 53.75±10.25, 54Control group 54.87±11.51, 52.5 | Chemotherapy and Abnoba VISCUMW Q (n=15) | Chemotherapy (n=14) | Adverse event (quality of life) |
Results of the search
Total 76 studies were selected in the initial search. Out of these, 45 were rejected due to non availability of data and duplicate records. Total 31 studies were screened with full text and later 17 studies were excluded due to incomplete clinical data. Non-availability of full text of the study was not considered as exclusion criteria. The study abstracts fulfilling the rest of the criteria were included as parameters like mean age, which were missing in the abstracts were not required for our analysis. Finally, 14 studies involving a total of 1965 participants (817 received various forms of phytomedicine or herbal medicine in addition to conventional therapy, and 1148 received conventional therapy only) suffering from various cancers (including cancers of the breast, prostate, nasopharynx, pancreas, stomach, ovary, non-small cell lung cancer and osteosarcoma), were included in this review [Table/Fig-2].
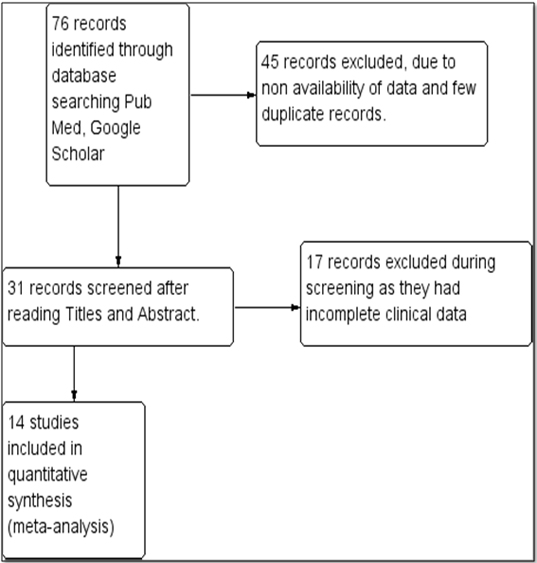
Risk of bias in the included studies
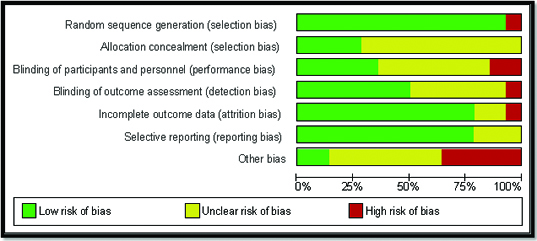
Overall, the included studies suggested low risk of bias as these studies generally had a randomized controlled, double-blind design, typically employing an Intention-To-Treat (ITT) analysis [Table/Fig-3]. Inter-rater agreement for the key quality indicators randomization, concealment of allocation and blinding was complete with no full publication necessary to be discussed by a third author. See [Table/Fig-4] for a summary of the judgments of the risk of bias for each domain in each of the included trials.
Methodological quality summary: review authors’ judgments about each methodological quality item for each included study
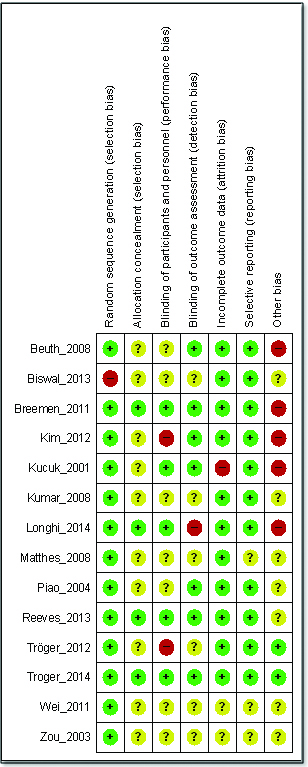
Methodological quality graph: review authors’ judgments about each methodological quality item presented as percentages across all included studies
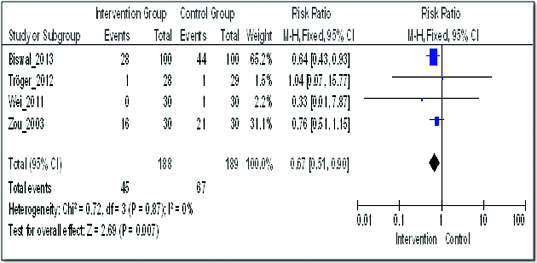
Mortality of the patients after one year of treatment for cancer
Four studies had compared the effect of addition of herbal medicine on mortality [11–14]. In comparison with conventional therapy (control arm), phytomedicine in combination with conventional therapy resulted in a significant reduction in mortality: Risk Ratio (RR) 0.67 (95% confidence interval (CI) 0.51 to 0.90) [Table/Fig-5]. These four studies were heterogeneous as the I2 was 0%.
Comparison of addition of phytomedicine to conventional therapy with the conventional therapy; outcome: mortality
Effect on Prostate Specific Antigen levels
On quantitative analysis of three studies for the effect of addition of herbal medicine on the levels of Prostate Specific Antigen (PSA) among patients with prostate cancer, the Standardized Mean Difference (SMD) with IV Fixed model was -0.70 (95% Confidence Interval (CI) -1.06 to -0.34). But, these clinical studies had substantial heterogeneity, with I2 of 82% (this can be explained by the difference in the control group and intervention group difference of these studies and the sample size of Reeves et al., is higher as compared to other two studies) [Table/Fig-6] [7,15,16].
Comparison of addition of phytomedicine to conventional therapy with the conventional therapy; outcome: prostate specific antigen levels
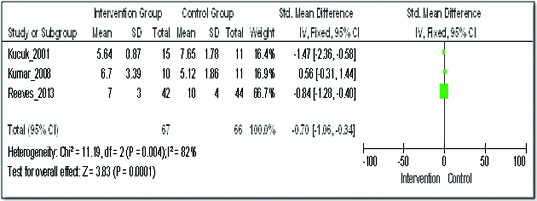
Effect on lycopene levels
On quantitative analysis of three studies for the effect of adding herbal medicine on lycopene levels among patients with prostate cancer, the standardized mean difference (SMD) with IV Fixed model was 1.55 (95% Confidence Interval (CI) 1.07 to 2.03) [Table/Fig-7] [7,15,16]. In the intervention group, plasma lycopene levels increased by 1.55 points as compared to the control group.
Comparison of addition of phytomedicine to conventional therapy with the conventional therapy; outcome: plasma lycopene levels
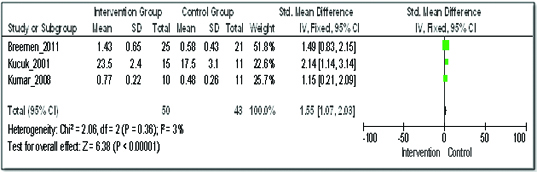
Adverse reactions: Two studies were analysed for the occurrence of adverse drug reactions with the therapy [18,19]. The combination of phytomedicine with conventional therapy resulted in a significant reduction in adverse drug reactions: RR 0.62 (95% CI 0.54 to 0.71). The two included studies have significant heterogeneity with I2 of 96% (this can be explained by the difference in the control group and intervention group differs of these studies especially Beuth et al.) [Table/Fig-8].
Comparison of addition of phytomedicine to conventional therapy with the conventional therapy; outcome: adverse events
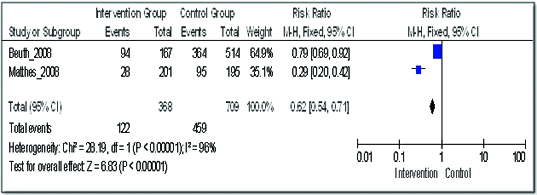
[Table/Fig-9] shows that the number of adverse events is comparatively less in the phytomedicine groups as compare to the control groups.
Incidence of adverse effects in the two treatment arms
| Study orStudy Group | Phytomedicine | Control |
|---|
| Events | Total patients (n) | Events | Total patients (n) |
|---|
| Piao 2004 | 57 | 115 | 100 | 108 |
| Beuth 2008 | 169 | 167 | 552 | 514 |
| Troger 2014 | 45 | 29 | 12 | 30 |
| Longhi 2014 | 16 | 9 | 69 | 11 |
| Kim 2012 | 92 | 15 | 96 | 14 |
| Total No. | 379 | 335 | 829 | 677 |
Cost effectiveness: [Table/Fig-10] shows the cost-effective analysis of addition of phytomedicine to cancer chemotherapy [23–26]. Addition of phytomedicine resulted in an increase in the annual cost of treatment by INR 1.241 Billion (US$ 19.64 Million) and prevented 25,217 deaths: the cost-effectiveness of phytomedicine is INR 49,237/death averted (US$ 779/death averted). The available epidemiological data was used to compute the cost-effectiveness.
Cost effectiveness analyses

Discussion
Similar to the upward trend of increasing incidence of cancer cases and deaths, the cost of cancer therapy is also escalating throughout the world, with India being no exception [6]. One important reason of rural indebtness is cancer treatment leading to poor care and high mortality [5].
The cost-effective analysis of cancer treatment is highly significant in the Indian set-up because of various reasons. Post 1990s, the economic growth has definitely occurred in the country, but, the Gross Domestic Product (GDP) per person is still low at about US$ 1500 [2]. India has a high mortality to incidence ratio as compared to countries with very high Human Development Index (HDI) and high HDI (68.6 vs 37.7 and 56.8) [2]. This implies that less than 30% of Indian patients with cancer will have survival of 5 years or longer after diagnosis. An important reason for this low percentage is late presentation of cancer patients for treatment. During the advanced stages, palliative care and pain relief have become more important part of the management [27]. Another important issue is the low budgetary allocation by the government for healthcare [2]. At present, cancer care in India is in its developing stage there is lack of involvement of primary health system in the cancer control activities and people are not aware about screening and the importance of early treatment. The cancer burden is distributed according to the socio-economic inequalities in the country.
In this scenario, various steps are being suggested for improvement of cancer care for the population. These include wider use of screening programmes, cost-effective vaccination and easy and cheaper availability of cancer treatment. The results of our study show that the addition of phytomedicine to the conventional therapy will be cost effective and thus can be considered another way of improving the cancer care.
The efficacy and safety of phytomedicine in cancer chemotherapy has been studied across the world. The clinical effect of phytomedicine when taken along with conventional treatment of cancer shows potential positive outcome and decreases adverse effects and also mortality. Various studies have shown beneficial effects of combined use of herbal products like mistletoe with the conventional therapy on the disease, therapy-related symptoms and quality of life among patients with breast cancer [8,18,20,28]. An invitro study has shown that Curcumin, increases the apoptosis of HepG2 cells, inhibits their proliferation and also causes elevation of caspase-3 activity [29]. Viscum album fermentatum Pini (Viscum), an extract of mistletoe led to better quality of life and longer ’post relapse disease free survival’ among patients with osteosarcoma free from disease after second metastatic relapse as compared to oral etoposide [21]. Another included study shows that Withania somnifera (Ashwagandha) root extract led to a significantly better quality of life by different measuring scales and a non-significant (p=0.176) longer 24- month overall survival [11]. Wei et al., showed that administration of Yanshu (a Chinese traditional medicine from Sopheraflauescens Ait) injection led to a significantly (p<0.05) higher quality of life and significantly (p<0.05) lower chance of myelo-suppression and thrombocytopaenia among patients with stage III nasopharyngeal carcinoma [13]. But, there are contradictory results as well. Hamilton-Reeves et al., found that short-term soy isoflavone administration might be beneficial in lowering hormone levels in patients with prostate cancer in a pilot study. Later, these results could not be proved in a randomised study of short duration [7]. However, the authors have recommended long-term studies with soy isoflavones as these might be involved with alterations in gene expression leading to beneficial results [30]. [Table/Fig-8] shows that phytomedicine increases the levels of lycopene which further help in decreasing the PSA levels among patients with prostate cancer. The results show that phytomedicine addition is not only limited to improvement in the quality of life and the tolerability of conventional treatment, but, these herbal medicines have their clinical efficacy as well.
Limitations
The inclusion of different types of cancers with different chemotherapy regimens makes the comparison among the studies difficult. We didn’t consider funnel plot as the studies for isolated outcomes were less. Also, the studies had small sample size. However, the strength of the study is the inclusion of studies with both low as well as unclear risk of bias, when it comes to the methodological quality of the studies [Table/Fig-3,4].
Conclusion
The results help in concluding that inclusion of phytomedicine into the cancer chemotherapy will be cost-effective and will help in decreasing the mortality and improving the quality of life of patients. Phytomedicine inclusion might help in lessening the socio-economic burden of India.