Cardiovascular disease (CVD) including stroke, heart attack and heart failure, is the leading cause of disease and death in the developed world, and is poised to become a significant health problem in developing countries [1]. Cardiovascular disease (CVD) accounts for 17.3 million deaths per year globally [2]. More people die of atherosclerosis and its complications, such as stroke, myocardial infarction, and arrhythmias, than all other medical problems combined [3]. Furthermore, cardiovascular disorders pose an increasing burden on health resources of many low and middle-income countries [4]. In the past few decades many treatment strategies have been developed based on the different pathomechanisms of CVD [5]. Inflammatory processes are important contributors to atherogenesis. Atherosclerosis is the dominant cause of cardiovascular disease. One of the most extensively studied biomarkers of inflammation in cardiovascular diseases is C-reactive protein (CRP), and high levels have been shown to predict cardiovascular events and appear to confer greater risk for cardiovascular disease [6]. Furthermore, people with type 2 diabetes also have high rates of high blood pressure, dyslipidemia, and obesity, which contribute to their high rates of CVD [7].
Diabetes mellitus Type 2, is a chronic condition leading to micro-vascular complications such as nephropathy, neuropathy, retinopathy and macro-vascular complications such as coronary heart disease, peripheral vascular disease and stroke [8]. A WHO 2009 report, ranks hyperglycaemia third, when examining deaths attributed to risk factors [1]. Type 2 diabetes is multi-factorial with genetic predisposition as well as environmental factors of diet and obesity [9]. There is a growing interest in herbal remedies due to some side-effects associated with conventional hypoglycaemic agents and increasing scientific evidence of the efficacy of some medicinal plants [10].
Furthermore, the use of medicinal plant preparations all over the world exceeds that of conventional drugs by two to three times [11]. Medicinal plants including Crataegus oxycantha, and Astragalus membranaceus, have been found to have therapeutic benefits for the treatment of cardiovascular disease [12].
The α-adrenoceptor agonist drug (Doxazosin) used for treating hypertension also appears to have therapeutic effects on benign prostatic hyperplasia (BPH) [13]. The root extract of Croton membranaceus Mull. Arg. (Euphobiaceae) has been used in the treatment of BPH and prostate cancer. Many of the same risk factors associated with CVDs also relate to risk factors for BPH. However, the full potential of C. membranaceus in offering protection against CVDs has not been explored. The primary aim of the study therefore was to investigate the anti-lipidemic and anti-inflammatory effects of C. membranaeceus using Spontaneously Hypertensive rats (SHR), a genetic model of hypertension that is widely accepted in medical research because of the features they share with idiopathic hypertension in humans [14]. The secondary aim was to examine its hypoglycaemic potential using diabetic mice.
Materials and Methods
Plant Material
The roots of Croton membranaceus were harvested in December 2012 and authenticated by the Center for Scientific Research into Plant Medicine (CSRPM), Mampong, Akuapem, Ghana. The sample of the plant was deposited at the herbarium of CSRPM with a voucher specimen number CSRP 2110. The aqueous root extract was obtained as previously described [15]. The freeze-dried extract was weighed and stored in a sealed container in a refrigerator at a temperature of 5 ± 3oC until use.
Two experiments were carried out: In experiment 1, male SHR were used to determine the hypolipidemic and anti-inflammatory effects of C. membranaceus aqueous root extract (CMARE). In experiment 2, diabetic mice db/db and db/+ were used to determine the hypoglycaemic effect of CMARE.
Animals
The protocol was approved by the Scientific and Technical committee of the Noguchi Memorial Institute for Medical Research (NMIMR) (STC 2009-02-3). SHR were provided by NMIMR and housed at the University of Ghana Medical School Animal Experimentation Unit in a temperature controlled room (25± 2°C) under a 12h light /12h dark cycle and allowed to acclimatize for 7 days prior to the studies. Diabetic mice were also housed at NMIMR under similar standard international housing conditions. Animals were treated humanely and fed the standard pellet diet and water ad libitium.
Experiment 1: Effect of CMARE on Lipid Profile and Inflammation
Male SHR were divided into four groups of 6 rats each. SHR in group I (normal control) fed the standard chow diet and water only. Rats in group II (low dose - LD) were orally administered with a dose of 25 mg/kg b.wt. of CMARE, rats in groups III (intermediate dose - ID) and IV (high dose- HD) were orally administered with 50 and 100 mg/kg b.wt. CMARE, respectively. All rats fed the standard chow diet. After 60 days of extract administration, rats were anesthetized {0.1 ml/100 g wt. ketamine hydrochloride and xylazine hydrochloride (4:1)} and cardiac puncture performed on all animals before euthanasia. 3ml of blood was dispensed into serum separator gel tubes. Serum was used for Triglycerides (TG), Total Cholesterol (TC), and High Density Lipoprotein cholesterol (HDL) using BioSystem kits, Calibrators and standards (Madrid, Spain). The assays were carried out according to the manufacturer’s instructions on the A25 BioSystem autoanalyser (Madrid, Spain). LDL cholesterol was calculated using the Friedwald equation {LDL= TC-HDL-(TG/2.2)}.
CRP Determination
High levels of C-reactive protein (CRP) have been shown to predict cardiovascular events and appear to confer greater risk for cardiovascular disease. Therefore the assessment of CRP gives an indication of the degree of inflammation. Serum was used for C-reactive protein (CRP) determination using i-CHROMATM kits (London, UK) on the i-CHROMATM reader (Gang-won-do, Republic of Korea).
In brief, i-CHROMATM CRP is based on fluorescence immunoassay. This technique uses a sandwich immune detection method that allows the fluorescence-labeled detector anti-CRP antibody in a buffer to bind to CRP antigens in the blood sample. The Ag-Ab complex when introduced onto a test cartridge and the complex allowed to migrate on the nitrocellulose matrix of the test strip by capillary action is captured by a second antibody recognizing the CRP Ag-Ab complex. Signal intensity of the fluorescence of the detector antibody read on the i-CHROMATM microprocessor unit, reflects the amount of CRP captured, hence the amount in the blood sample. A CRP control was used for quality control.
Experiment 2: Effect of CMARE on Glycaemic Activity
Adult db/db and db/+ male mice weighing 30.5-46.5 g and 5-7 months old bred in NMIMR (Department of Animal Experimentation) were used for the experiment. The strains db/db mice were used for the test groups and positive controls, whereas db/+ mice were used for the negative controls. Mice were kept in steel cages within the facility and allowed free access to water and standard mice pellets. On transfer to the research area, mice were allowed one week acclimatization period before commencement of the experiment. The mice were deprived of food 12 hours prior to the experiment.
Pre-experiment Screening
At the commencement of the experiment, 32 db/db mice were weighed using the Animal Scale TS 870 (TGC INT, Japan). The tails of mice were cleaned with 70% ethanol and allowed to dry. The level of Fasting Blood Glucose (FBG) of the mice was determined using Lifescan Johnson and Johnson One Touch Ultra® glucometer kit and One Touch Ultra 2 glucometer (Milipitas, CA) after obtaining blood by tail tip pricking. The db/db mice with FBG< 9.0 mmol/l were then excluded from the experiment whereas db/db mice with FBG > 9.0 mmol/l were used for the experiment. These diabetic mice (totaling 21) were then randomly selected into three groups (7 per group), Croton group, Metformin group, and the Positive control group. The Negative control group db/+, was made up of 7 non-diabetic mice. Mice were individually labeled and handled according to the International Convention for the use and care of experimental animals [16].
Glycaemic Activity Test
CMARE and metformin tablets were dissolved in distilled water and orally administered at a dose of 250 mg/ kg b. wt. to the respective mice groups using calibrated syringes and ball-ended administrative needles. The positive and negative control groups were however not administered with either CMARE or metformin but with an equivalent volume of distilled water. Mice were allowed to feed continuously following CMARE, metformin or distilled water administration and blood glucose level (BGL) was determined by tail vein bleeding at 1, 2, 3 and 15 hours.
Statistical Analysis
Data obtained was analysed using SPSS (Statistical Package for Social Sciences) version 20.0. Means ± SEM were determined for quantitative variables. To determine the existence of statistical significance, student t-test (paired) was used while variables with more than two outcomes, analysis of variance (ANOVA) was used followed by Bonferroni post-hoc test. A p-values ≤ 0.05 were considered significant.
Results
Effect of CMARE on Lipid Profile
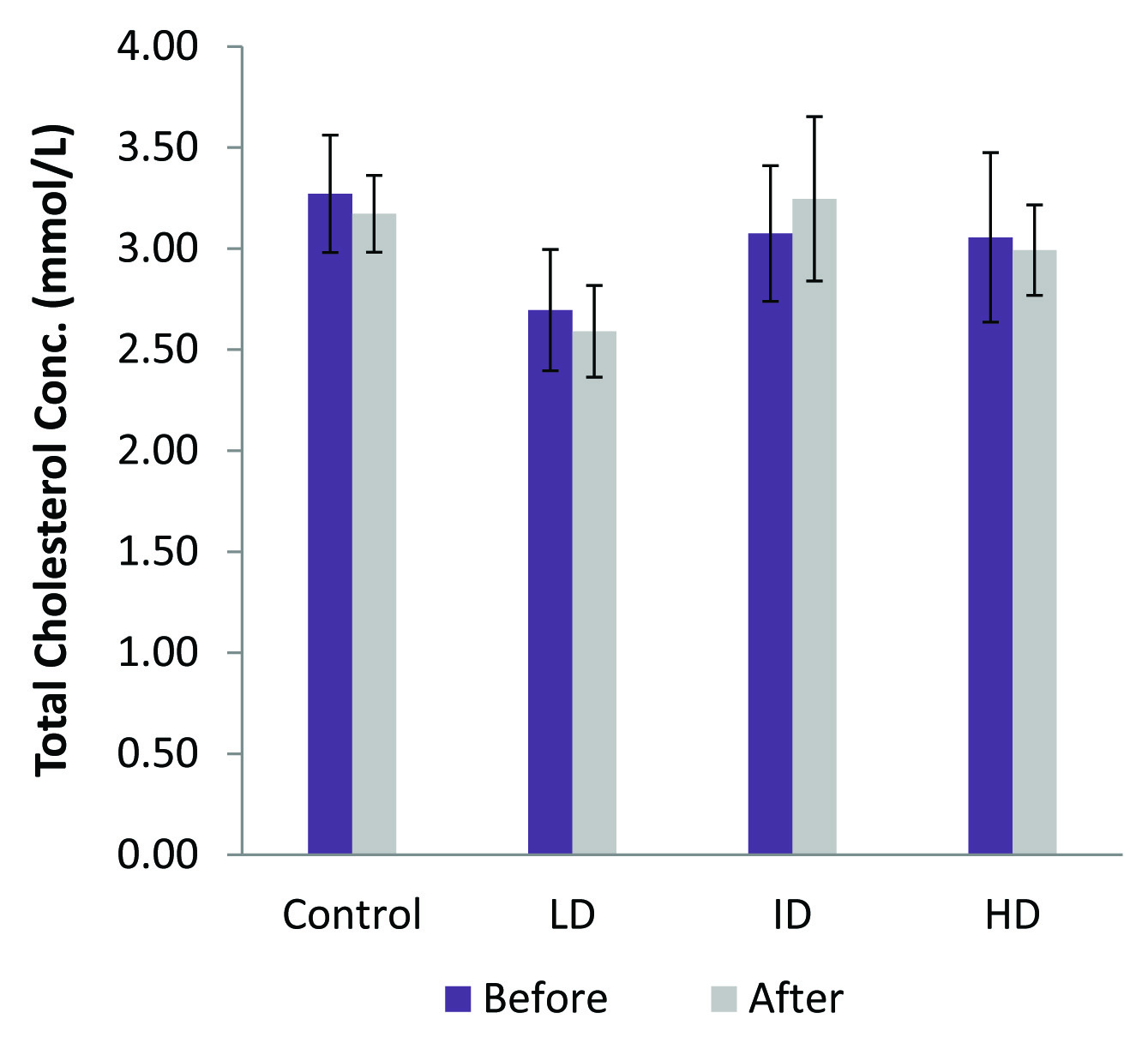
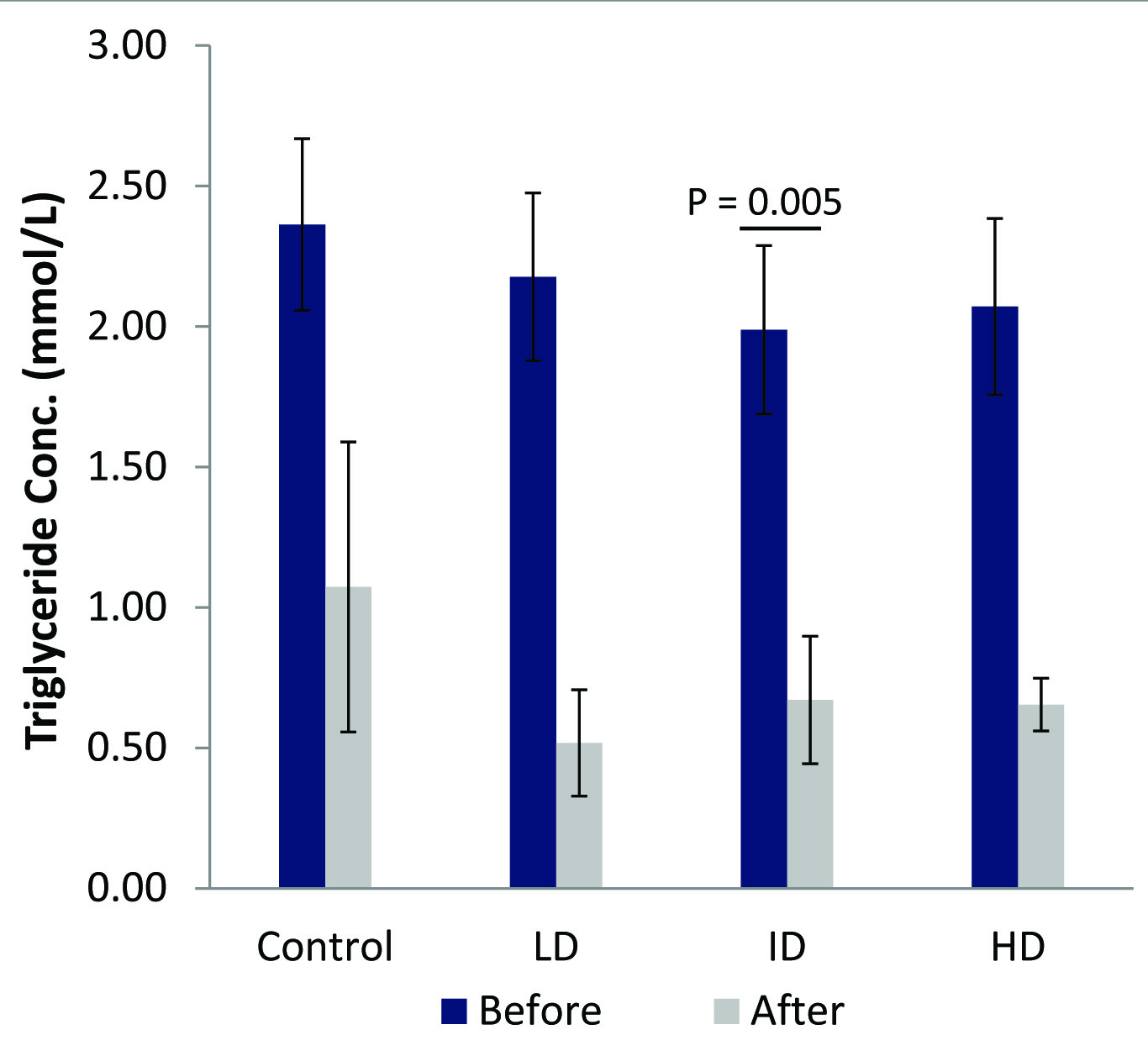
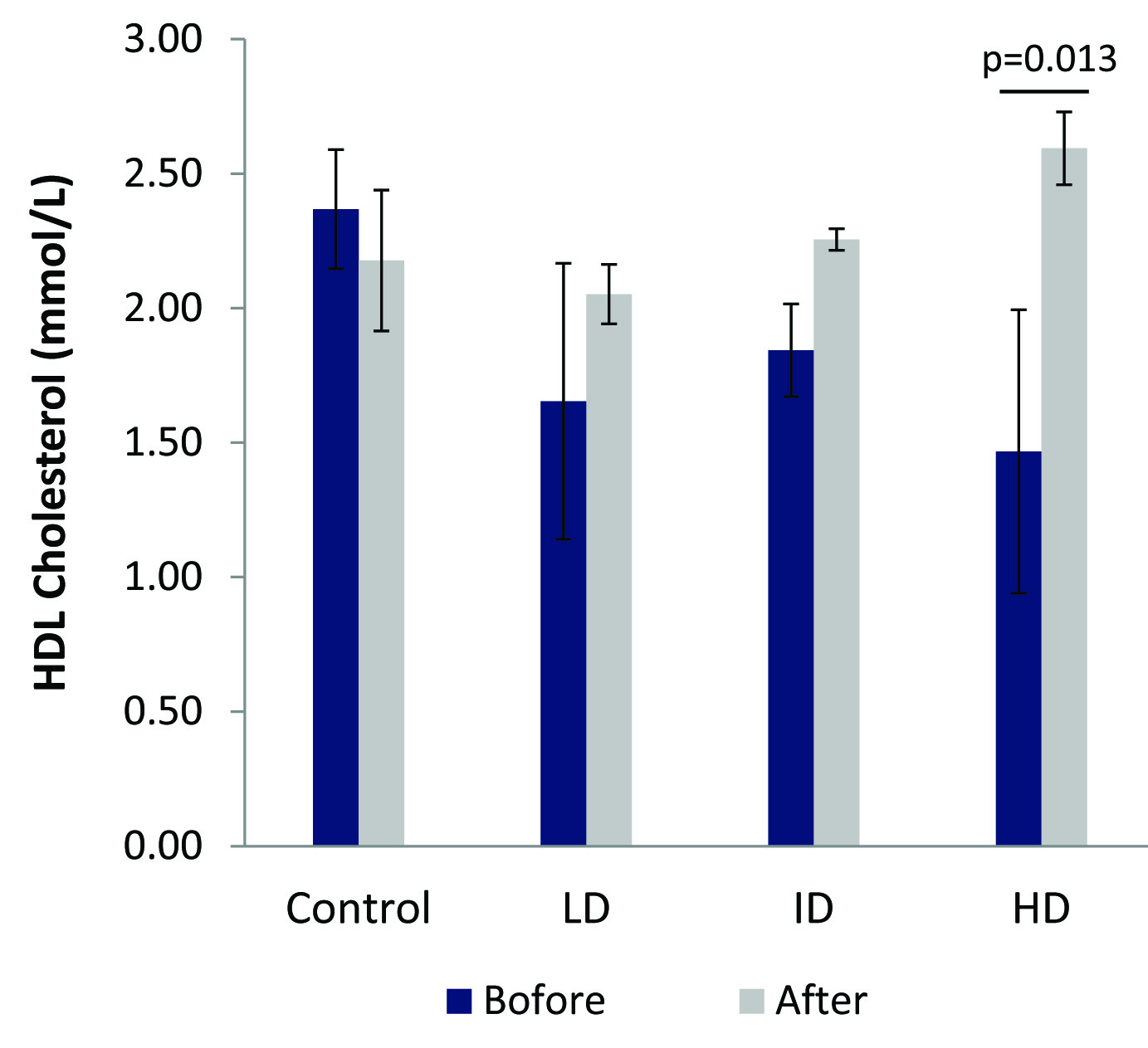
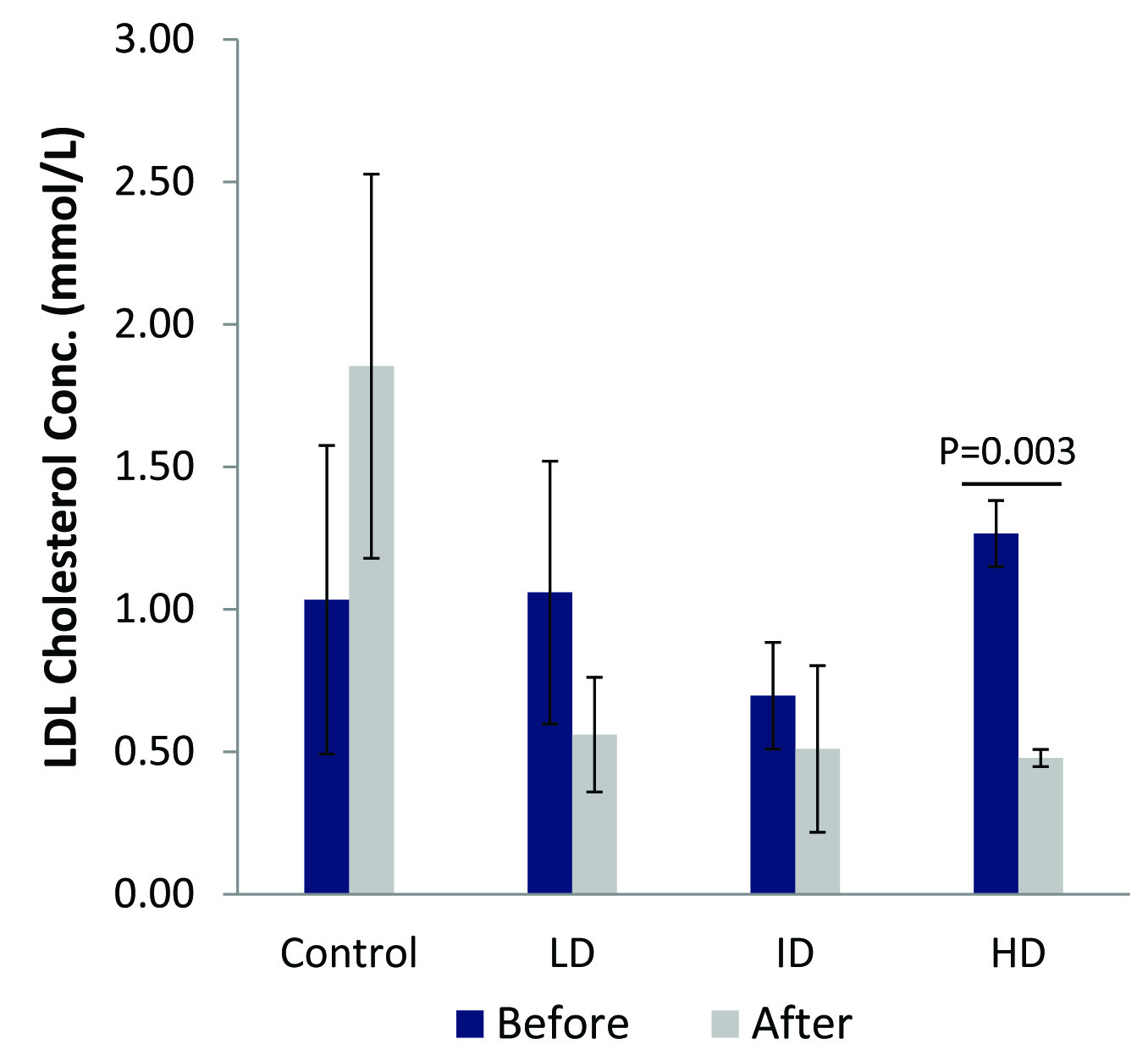
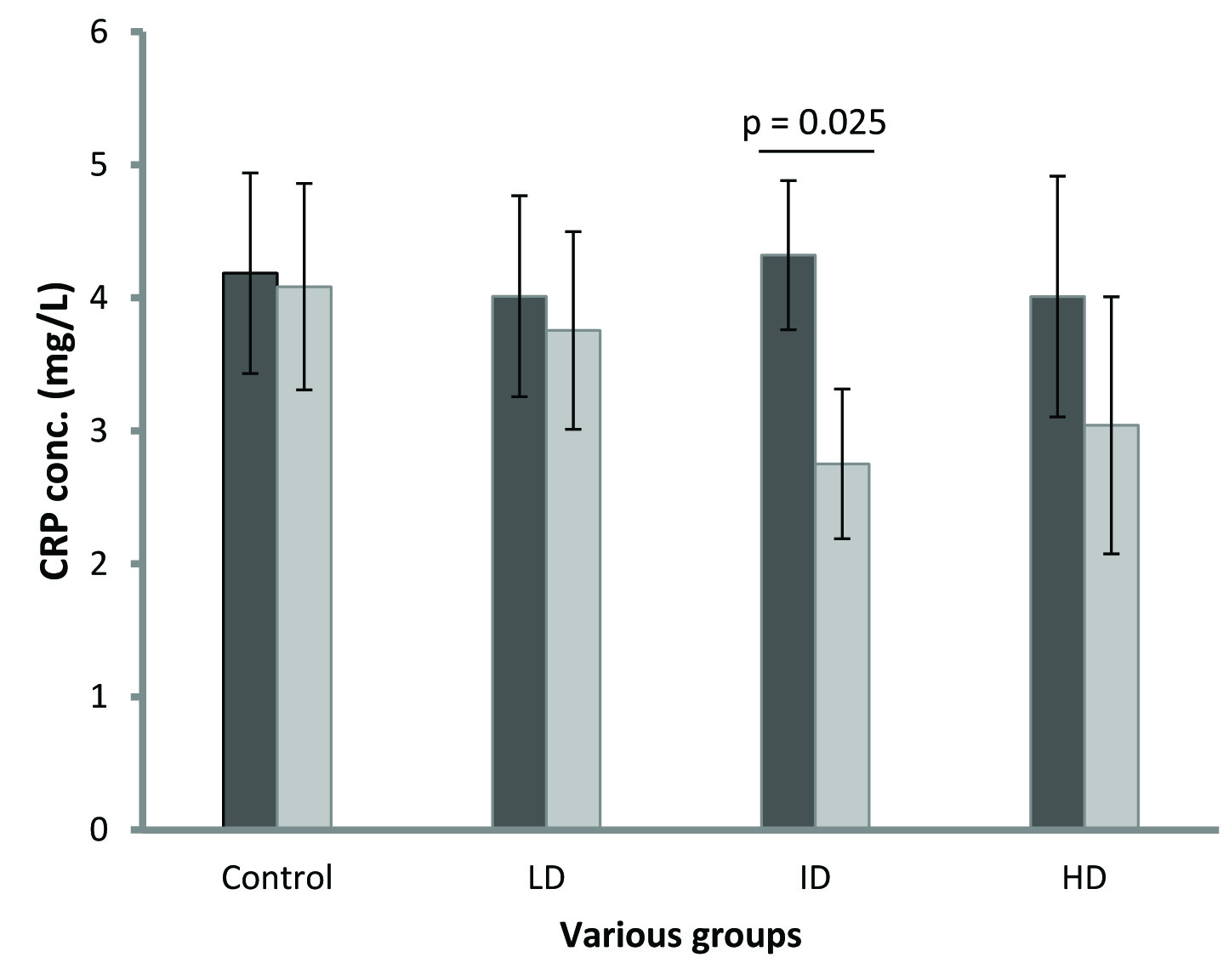
In the low dose group there was no significant change in any of the analytes estimated. TC did not show any significant change among the various groups [Table/Fig-1]. In the intermediate group, a significant hypotriglyceridaemic effect (p=0.005) was observed [Table/Fig-2]. In the high dose group, HDL and LDL showed a significant increases (p=0.013) and decrease (p=0.003), respectively [Table/Fig-3,4]. ANOVA between the four groups for HDL, LDL, TG and TC were all not significant. However, a post-hoc analysis (LSD) showed a significant association between the control and high dose LDL (p=0.021) and control and high dose HDL (p=0.046). CRP values before and after for the four groups were as follows: C = 4.20 ± 0.33 and 4.1 ± 0.35; LD = 4.0 ± 0.34 and 3.76 ± 0.33; ID = 4.3 ± 0.25 and 2.75 ± 0.25; HD = 4.01 ± 0.41 and 3.0 ± 0.43. All results were expressed in mg/l. Significant differences were observed in the ID and HD groups (p=0.010, p = 0.011, respectively) [Table/Fig-5].
Total cholesterol (TC) levels of the low dose (LD), intermediate dose (ID) and high dose (HD) groups did not show any significant change after 60 days of CMARE administration to spontaneously hypertensive rats (SHR)
Significant triglyceride (TG) change (p=0.005) was observed in the Intermediate dose group (ID) after 60 days treatment with 50 mg/kg b.wt. CMARE administration to male spontaneously hypertensive rats (SHR)
HDL cholesterol significantly increased (p=0.013) in the high dose group (HD) after 60 days of 100 mg/kg b, wt. CMARE administration to spontaneously hypertensive rats (SHR)
A significant difference (p=0.003) in LDL cholesterol was observed in the high dose group (HD) after 60 days of 100 mg/kg b.wt. CMARE administration to male spontaneously hypertensive rats (SHR)
The figure show reduction in CRP after SHR were orally administered low dose (LD = 25 mg/kg b.wt), intermediate dose (ID=50 mg/kg b.wt) and high dose (HD = 100 mg/kg b.wt) CMARE for 60 days. Mean CRP levels for the 50 mg/kg b.wt. group were significant after treatment (p = 0.025)
Effect of CMARE on Glycaemic Activity
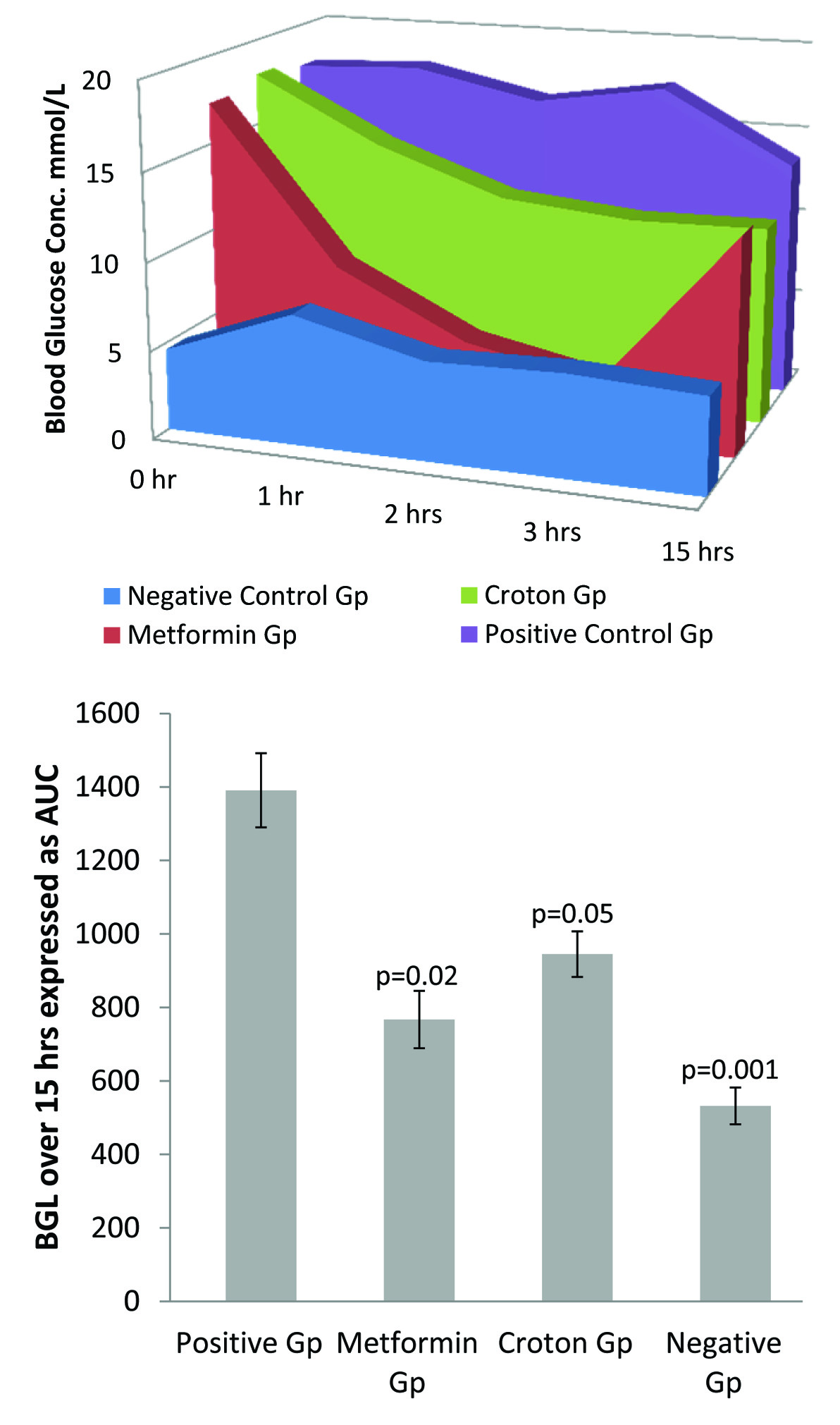
At 0 h there was no significant difference in fasting blood glucose (FBG) in both the Metformin and Croton groups compared to the positive control group. At 1 hours, there was a significant reduction in BGL in the Metformin group compared to Positive control group (p= 0.000), however, there was no significant reduction in the BGL of the Croton group compared to the Positive control group. At 2 hour there was a significant reduction in BGL in the Metformin group compared to the Positive control group (p= 0.000), whereas for Croton group there was no significant reduction in BGL compared to the positive control group (p=0.083). Contrary to the above trend however, there was a significant reduction of the BGL in both the Metformin and Croton groups (p=0.000; p= 0.006, respectively) compared to the positive control group at 3 hours. Additionally, at 15 hours, BGL in the Croton group was 11.2 ± 1.56 mmol/l which was lower than the level in the Metformin group (12.31 ± 1.63 mmol/l). However, differences were not significant compared to the positive control group. The negative control demonstrated the normal pattern of postprandial glucose levels. Area under the curve (AUC) demonstrated significant hypoglycaemic effect of CMARE compared to the positive control [Table/Fig-6].
Bar chart [Table/Fig-6b] of AUC [Table/Fig-6a] showing significantly reduced blood glucose level (BGL) in the db/db mice treatment groups {Metformin Gp - 250 mg/kg b.wt. metformin) (p=0.02) and Croton Gp – 250 mg/kg b.wt. CMARE (p=0.05)} compared to the untreated group (positive control) after 15 hours of single dose administration. However, differences between treatment groups and negative control db/+ mice, were significant after 15 hours (p=0.001)
Discussion
Spontaneously hypertensive rats (SHR) are excellent models of primary or essential hypertension that occurs in humans. This model of cardiovascular disease has been used extensively in over 4000 Medline references [17]. SHR follow the same progression of hypertension in humans starting from pre hypertension to a sustained hypertension phase with each lasting several weeks [18].
The combined abnormalities of lipids such as raised total cholesterol, low High-Density Lipoprotein cholesterol (HDL), and Low-Density cholesterol lipoprotein (LDL) as well as their independent variables have also been suggested to characterize hypertensive dyslipidemia, and either of these abnormalities is independently atherogenic. Bay leaves decreased LDL cholesterol by 32 to 40%, HDL cholesterol increased by 20 and 29% and TG also decreased by 25-34% after 30 days of capsulated powdered leaves administration [19]. Other studies have also shown that treatment with dandelion (Taraxacum officinale) root, positively changed lipid profiles in cholesterol-fed rabbits, suggesting potential hypolipidemic effects [20]. In some studies the root back extract of Nauclea latifolia not only lowered the LDL and HDL levels positively, but also reduced cholesterol levels [20]. Roots of Asparagus racemosus (AR) widely used in Ayurvedic system of medicine in India is known for its steroidal saponin content. The root extract reduces cholesterol levels by stimulating bile production, which in turn binds cholesterol [20].
In this aspect of the study, the significant effect of CMARE was seen in the HD group. TC levels remained relatively unchanged at 3.0 mmol/l. This represents a much higher level compared with earlier studies on SHR (1.46 mmol/l) [21]. LDL was significantly reduced from 1.27 to 0.48mmol/l (p = 0.003) in the HD group. This was much higher than results obtained from the study of Tomiyasu et al., (LDL = 0.12 mmol/l) after SHR fed Hippophae rhamnoides for 60 days [22]. Furthermore, HDL (high dose group) significantly increased from 1.47 to 2.59mmol/l (p = 0.013) contrary to the decrease that was observed in the study of Tomiyasu et al., [22]. HDL increase is cardiovascular protective. TG in the ID group was initially 1.99 and reduced to 0.67mmol/l after 60 days which was statistically significant (p = 0.005). Thus, TG levels dropped by 66%. Similar results were obtained by Singh et al., using Cynodondactylon extract in streptozotocin diabetic rats. In that study, TG reduced by 77% [23].
One biomarker of cellular events during inflammation is CRP. Few medicinal plants have been implicated in lowering CRP. In this study, CRP was lowered in C. membranaceus intermediate dose-treated group. Similarly, the Chinese monoherbal injection lowered inflammatory markers including CRP when the inflammation induced by carrageenan in the rat pleurisy model, and by xylene in the mice ear edema model, were adopted to study the anti-inflammatory activity of Houttuynia cordata [24]. Other inflammatory markers may be employed in future studies to affirm the anti-inflammatory properties of C. membranaceus.
The incidence of CVD is increased 2-4 fold in people with type 2 diabetes [25]. Several therapies for reducing hyperglycaemia are currently available. These include treatment by sulfonylureas that stimulate pancreatic islet cells to secrete insulin, α-glucosidase inhibitors that interfere with glucose absorption and metformin which acts to reduce hepatic glucose production. However, because of the numerous side effects and limited efficacy, alternative therapies are being sought all over the world. Most plant extracts have several activities against various ailments and have multiple target sites. In this study CMARE reduced BGL from 17.99 to 11.07 mmol/l, 3 hour after administering CMARE. Levels were significantly lower than values in diabetic mice. This significantly mild hypoglycaemic activity was sustained at a much longer period than the effect of metformin. Indeed at 15 h, values in the CMARE and metformin groups were 11.21 and 12.31 mmol/l, respectively, suggesting a sustained hypoglycaemic effect of CMARE, against the rapid hypoglycaemic effect of metformin, hence the less tendency for CMARE to cause hypoglycaemia in diabetic patients. The bark of Croton cajucara Benth. (Euphorbiaceae) demonstrated a significant hypoglycaemic activity in alloxan-induced diabetic rats, at oral doses of 25 and 50 mg/kg body weight [26]. Similarly, the stem barks of Croton cuneatus Klotz (Euphorbiaceae) had an antidiabetic activity in hyperglycaemic rat models [2]. While the leaves of most hypoglycaemic medicinal plants such as Croton zambesicus have been documented, only few plants have such activities in the roots [27]. Others like Biophytum sensitivum (Oxalidaceae), and Carallumaedulis (Apocynaceae) stimulate the synthesis and release of insulin from the pancreas [28]. Yet others like Calamintha officinalis Moench (Lamiaceae) cause hypoglycaemia without affecting insulin levels [29]. For other plants such as Cassia auriculata (Fabaceae) there is a shift from gluconeogenesis to glycolysis [30]. The exact mechanism by which C. membranaceus exerts its hypoglycaemic effect is yet to be elucidated.
Limitation
One animal model representative of cardiovascular disease and diabetes would have been the ideal model instead of the two different animal models used.
Conclusion
In conclusion, the findings of this study revealed that CMARE significantly lowered triglyceride, CRP and glucose levels and may be apposite in dealing with some aspects of CVD in addition to its major therapeutic effect on BPH.