Lower dietary zinc intake and lower concentrations of serum zinc might be associated with a higher risk of cardiovascular diseases, diabetes and glucose intolerance [1,2]. The relationship between zinc and metabolic health status is controversial. Several studies have reported that higher zinc intakes have protective effects against metabolic syndrome [3,4]. A large population based prospective study revealed that higher zinc intake might associated with lower risk of type 2 DM in women [5]. A lower serum zinc levels in patients with DM have been observed in other population [6]. However, others have reported that the serum zinc concentrations do not differ according to glucose tolerance [7,8]. Evidence for the casual role of zinc in the development of diabetes is also controversial, but there is clear evidence for the increased secretion of insulin [9].
Materials and Methods
Individuals and Study Design
A cross-sectional study was carried out during the period from April to October 2014. Three hundred and thirty one apparently healthy individuals were included (190 males and 141 females). Of these individuals, 201 were siblings of patients with type 2 DM. The remainder (n=130) were apparently healthy individuals without family history of type 2 diabetes chosen from the relatives of patients attending Azadi General Teaching Hospital in Duhok, Kurdistan Region, Iraq, served as a control group. Protocol involved that: all patients diagnosed as type 2 DM or being treated as such who visited the Duhok Diabetes Center; during the period of the study (n=5783) were interviewed and informed about the nature of the study, and then asked to bring their siblings who are at the age range of 20-40 years in fasting state. At the beginning, a total of 286 were participated in the study. After exclusion of 85 responders who were with BMI >27 or <20 Kg/m2, non-fasting, women with pregnancy, as well as those who had an acute infection that required current antibiotic therapy and those who were taking medication that altered zinc metabolism or state, the reminders were enrolled in this study. The study protocol was approved by the ethical committee of the General Directorate of Health, and informed consent was obtained from all the participants at the start of study.
Data Collection: Data were collected from individuals interviewed by special questionnaire form. Included data were demographic information about the individual (age and sex), personal history of DM, coronary heart disease (angina, myocardial infarction), hypertension, weight gain (after 18 years in women, 21 years in men), low birth weight (<2.5 Kg), and gestational DM and menstrual irregularities (oligomenorrhea or amenorrhea) in women, and family history of type 2 DM, premature coronary heart disease (CHD), essential hypertension (<60 years) and hypertriglyceridemia.
Then physical activity was assessed by asking about the physical activity (work, leisure, and travel) in a typical week. History of hypertension was defined as blood pressure > 140/90 mm Hg or being on an antihypertensive medication. Family history of premature CHD was defined as definite MI or sudden death in a first degree relative before 60 years of ages. MONW or non-MONW individual was define according to the scoring method for identifying an MONW individual represented by Ruderman et al., [13]. The proposed scoring value for identifying an MONW individual was > 7. Waist circumference was checked by using a plastic metric tape applied midway between the lower costal margin and the iliac crest. Resting systolic and diastolic BP was measured by using random zero sphygmomanometer and cuffs appropriate for arm size. Biochemical blood measurements (HDL-cholesterol and triglycerides) were determined by a standard laboratory procedure using Cobas 6000. Roche/Hitachi. Serum concentration of insulin was measured by enzyme linked immunosorbent assay (ELISA) method. The homoeostasis model assessment estimates insulin resistance (HOMA-IR) was calculated using the following formula (Glucose {mg/dl} ×Insulin {uIU/ml})/405. Serum zinc was analysed by flame atomic absorption spectrophotometer (Perkin Elmer) using a standardized procedure.
Assessment of zinc status
Zinc status was assessed as follow: Severe zinc deficiency (serum Zinc < 50 μg /dl), marginal zinc deficiency (serum Zinc 50-<70 μg /dl), Normozincaemia: serum zinc concentration > 70 -130 μg /dl and Hyperzincaemia (serum zinc >130 μg /dl).A cut off point of <70 μg /dl of Zinc was used to classify individuals as on low zinc status [14].
Statistical Analyses
All data was analysed using the statistical package for Social Sciences (SPSS); version 21.0. Independent t-test was used to assess differences in serum analyte among groups. Categorical variables were analysed by Chi-square tests.
Results
The general characteristics of the study individuals have been described in [Table/Fig-1]. The siblings of patients with type 2 DM exhibited significantly lower serum zinc level than did the healthy controls (p= 0.016). While the mean values of fasting blood glucose, insulin, HOMA-IR and triglycerides were significantly higher; and HDL-cholesterol was lower in the siblings compared with the controls. The other parameters, including age, blood pressure, BMI, and WC were similar in siblings and controls.
Siblings of patients with type 2 DM and control characteristics Results are Mean±SD
| Characteristics | Siblings(n=201) | Controls(n=130) | p-value |
|---|
| Age (years) | 29.0±5.2 | 28.6±5.7 | 0.845 |
| Male sex, n(%) | 95(47.3) | 95(73.1) | 0.013 |
| Systolic BP (mmHg) | 113.1± 9.3 | 115.3± 6.1 | 0.199 |
| Diastolic BP (mmHg) | 72.3 ±7.7 | 72.5± 7.0 | 0.894 |
| BMI (Kg/m2) | 24.0± 1.7 | 23.8 ±1.6 | 0.696 |
| Waist Circumference (cm) | 87.2 ±5.6 | 85.5±8.7 | 0.162 |
| Fasting blood glucose(mg/dl) | 101.7 ±17.8 | 93.8± 8.7 | 0.018 |
| Serum insulin (uIU/ml) | 7.06 ± 4.12 | 5.12±3.71 | 0.021 |
| HOMA-IR | 1.77±0.18 | 1.18±0.08 | 0.002 |
| Triglycerides (mg/dl) | 136±87.9 | 106±42.2 | 0.020 |
| HDL-Cholesterol (mg/dl) | 43.9±12.0 | 48.8±13.1 | 0.039 |
| Serum zinc (ug/dl) | 94.1±20.8 | 103.2±15.6 | 0.016 |
Of the two hundred and one siblings of patients with type 2 DM, 28(13.9%) had marginal hypozincaemia (serum zinc<70 ug/dl) as compared to 7/130 of the healthy controls (5.4%). None of the enrolled individuals had severe hypozincaemia [Table/Fig-2].
Zinc status in siblings of patients with type 2 DM and controls
| Zinc status | Siblingsn (%) | Controlsn (%) | p-value* |
|---|
| Severe zinc deficiency (<50 ug/dl) | 0 (0.0) | 0 (0.0) | - |
| Marginal Hypozincaemia (50-70 ug/dl) | 28(13.9) | 7(5.4) | 0.035 |
| Normozincaemia (>70-129 ug/dl) | 164(81.6) | 120(92.3) | 0.170 |
| Hyperzincaemia (>130 ug/dl) | 9(4.5) | 3(2.3) | 0.234 |
*Chi-square test
The mean and SD of serum zinc level with respect to metabolic syndrome components is demonstrated in [Table/Fig-3]. A Statistically significant difference was found in the mean value of serum zinc, using a p-value of 0.05 for body mass index, waist circumference, fasting blood glucose and triglycerides.
Serum zinc levels according to metabolic syndrome components in siblings of patients with type 2 DM
| Serum zinc level (ug/dl) |
|---|
| n | Mean±SD | p-value |
|---|
| Blood pressure> 140/90 mmHg | | | |
| Yes | 10 | 93.8± 20.4 | 0.566 |
| No | 191 | 94.1±20.8 | |
| Body Mass Index (kg/m2) | | | |
| >23 | 158 | 92.7±21.0 | 0.026 |
| <23 | 43 | 99.2± 20.0 | |
| Waist circumference (cm) | | | |
| Males>86.4, females>71.1 | 146 | 92.6 ±20.8 | 0.049 |
| Males<86.4, females<71.1 | 55 | 98.0±20.6 | |
| Fasting blood glucose (mg/dl) | | | |
| >110 | 36 | 63.8±20.7 | 0.014 |
| <110 | 165 | 100.7±20.8 | |
| Triglycerides (mg/dl) | | | |
| >150 | 55 | 80.5± 19.5 | 0.034 |
| <150 | 146 | 99.2 ± 21.3 | |
| HDL-cholesterol (mg/dl) | | | |
| <35 | 51 | 95.7±23.8 | 0.560 |
| >35 | 150 | 93.6±19.8 | |
The mean and SD of serum zinc level in MONW and non-MONW individuals has been shown in [Table/Fig-4]. Those categorized as MONW 85.1% exhibited lower serum zinc levels than did the non-MONW, but the difference was not significant (p=0.059).
Serum zinc level of the siblings of patients with type 2 DM categorized to MONW and non-MONW
| Characteristics | MONW | Non-MONW | p-value |
|---|
| Age (years) | 29.0 ± 5.2 | 26.9 ± 4.7 | 0.05 |
| Male sex, n (%) | 82 (48.2)* | 12(40.0) | 0.467 |
| Systolic BP (mmHg) | 113.0±9.2 | 113.3±10.0 | 0.912 |
| Diastolic BP (mmHg) | 72.0±7.5 | 73.8±9.0 | 0.911 |
| BMI (Kg/m2) | 24.2±1.6 | 22.8±1.5 | 0.013 |
| Waist Circumference (cm) | 87.3±7.7 | 75.8±7.3 | 0.001 |
| FBG (mg/dl) | 102.2±18.7 | 99.0±12.2 | 0.152 |
| Serum insulin (uIU/ml) | 7.2±4.6 | 6.3±3.7 | 0.057 |
| HOMA-IR | 1.81±1.05 | 1.54±0.81 | 0.045 |
| Triglycerides (mg/dl) | 146.7±35.3 | 77.5±35.7 | 0.001 |
| HDL-Cholesterol (mg/dl) | 43.2±11.7 | 48.1±12.8 | 0.049 |
| Serum zinc (ug/dl) | 93.7±20.9 | 96.2±18.1 | 0.059 |
Results are mean±SD., *Chi-square test
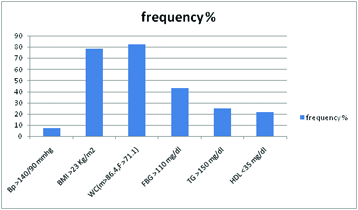
The association between serum zinc levels and the metabolic syndrome components in the MONW siblings were analysed [Table/Fig-5]. On using the Pearson’s correlation coefficient (r), the results showed a significant inverse relationship between serum zinc level and fasting blood glucose (r=-0.233, p<0.05). An inverse relationship was also found between serum zinc concentrations and triglycerides (r= -0.16, p=0.022). The correlations between serum zinc concentrations and blood pressure, body mass index, waist circumference and HDL-cholesterol were not statistically significant (r=0.035, r= 0.044, r= 0.045 and r=0.077), respectively. However, when the association between low serum zinc levels (<70 ug/dl) and the frequency of metabolic syndrome components were analysed, individuals with WC (M>86.4, F>71.1) cm had a higher percentage of marginal hypozincaemia as compared to the other components [Table/Fig-6].
Results of Pearson correlation co-efficient analysis demonstrating the association between serum zinc levels and metabolic syndrome components
| Components | r | p-value |
|---|
| Blood pressure >140/90 | 0.035 | 0.623 |
| BMI >23 (Kg/m2) | 0.044 | 0.539 |
| Waist circumference >86.4(cm) | 0.045 | 0.528 |
| FBG >110 mg/dl | -0.233 | 0.001 |
| Triglycerides >150 mg/dl | -0.162 | 0.022 |
| HDL-cholesterol <35 mg/dl | 0.077 | 0.279 |
Association of metabolic syndrome components with hypozincaemia in siblings of patients with type 2 DM
Discussion
This study has provided definitive evidence that siblings of patients with type 2 DM had low zinc status. The best association of low serum zinc levels was with obesity waist circumference, fasting blood glucose and triglycerides. Marginal hypozincaemia appears to be highly prevalent in the siblings (13.9%) compared to 5.3% in the healthy controls. It is therefore, such a prevalence of low zinc status in the siblings is especially noteworthy because several factors are known to impact negatively on zinc status. Of these, can be insufficient intake of dietary zinc. Mild to moderate zinc deficiency is common in several developing countries, because the commonly consumed staple foods have low zinc contents and are rich in phytates. The phytate contents of cereal proteins is known to decrease the availability of zinc, thus the prevalence of zinc deficiency is likely to be high in a population consuming large quantities of cereal proteins. However, dietary and non-dietary factors are observed to impact negatively on serum zinc concentration [15–17]. Therefore, the reduction in the mean serum zinc concentration in population reported here appears to be associated, at least in part, with low intakes of poorly available dietary zinc [18]. But in fact, this low zinc status (serum zinc <70 ug/dl) is highly associated with the metabolically unhealthy status in siblings. It is noteworthy that the prevalence of MONW individuals among the siblings of patients with type 2 DM was 85.1 % and most of the siblings (72.6%) was with obesity waist circumference>86.4 cm for males, and 71.1 cm for females. Moreover, they had higher means of HOMA-IR than controls, while the level of serum zinc was lower in those with high levels of blood glucose and triglycerides. A recent report from the Hunter Community Study revealed that high serum zinc concentration is associated with increased insulin sensitivity [9]. Considering the differences in risk factors across countries, diabetic patients have significantly lower mean serum zinc levels compared with non-diabetic and zinc supplementation for type-2 diabetics has beneficial effects in elevating their serum zinc level, and in improving the glycemic control that is shown by decreasing their HbA1c% concentration and fasting glucose levels [19]. These finding is a positive step towards further research to determine if zinc supplementation in siblings of patients with type 2 DM may reduce the risk of developing diabetes, this withstanding that 36/201(17.9%) of the study siblings was pre-diabetes or diabetes (FBG>110 mg/dl) and 27.4% had hypertriglyceridemia. A low serum zinc concentrations in metabolic syndrome has been suggested [20]. In the present study, the serum zinc levels were lower in siblings with increased body mass index, waist circumference, Fasting 5.4% blood glucose and triglycerides, which confirmed the relationship between zinc levels and prevalent metabolic syndrome [21].
There have been reports of prevalence of MONW in general population, which ranges from 5-45%. But, however none of these reports directly related the prevalence of MONW among siblings of patients with type 2 DM [22]. Thus, we carried out this cross-sectional study on three identities; MONW, zinc, and siblings of patients with type 2 DM. The prevalence of metabolic abnormalities for MONW was significantly higher than that of non-MONW. For example, MONW individuals had higher mean age, body mass index, obesity waist circumference than non-MONW group. MONW individuals had also higher mean values of serum triglycerides, but lower HDL-cholesterol and serum zinc concentrations. This finding agrees with trials performed in general population [23] as well as in our population [24].
Limitations
This study has few limitations, first we conducted this present study in Duhok Diabetes Center which is a health facility and health facility based studies are more likely to be biased than population based randomized studies regarding sampling. Second, this study is a cross-sectional study and a cross-sectional analysis has limitations as research methodology as it lacks follow up so the data presented are less likely to be representative of the general population actual data and of the same individual at other times. Third, some of the variables present in the study were depending on history taking and this carries an inherent risk of bias. Despite these limitations, our descriptive study interpreted with suitable caution can offer some useful insight to complement the data from the forthcoming studies using randomization
Conclusion
A low zinc status exhibited in 13.9% of the siblings of patients with type 2 DM, particularly among MONW individuals. This finding may have clinical implications due to the increased risk of future metabolic disease. A large prospective study is needed to confirm our observation, and experimental data may further elucidate the biological mechanism of the associations.
*Chi-square test
Results are mean±SD., *Chi-square test