Architectural Analysis of Picrosirius Red Stained Collagen in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma using Polarization Microscopy
Rashi Sharma1, Shweta Rehani2, Monica Mehendiratta3, Priyanka Kardam4, Madhumani Kumra5, Yulia Mathias6, Jyoti Yadav7, Khushboo Sahay8
1 Senior Lecturer, Department Oral Pathology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Haryana, India.
2 Reader, Department of Oral Pathology, Sudha Rustagi College of Dental Sciences & Research, Faridabad, Haryana, India.
3 Reader, Department of Oral Pathology, ITS Dental College, Greater Noida, Uttar Pradesh, India.
4 Senior Lecturer, Department of Oral Pathology, Sudha Rustagi College of Dental Sciences & Research, Faridabad, Haryana, India.
5 Professor, Department of Oral Pathology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Haryana, India.
6 Professor and Head, Department of Oral Pathology, Sudha Rustagi College of Dental Sciences & Research, Faridabad, Haryana, India.
7 Senior Lecturer, Department of Oral Pathology and Mirobiology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, India.
8 Senior Lecturer, Department of Oral Pathology, Sudha Rustagi College of Dental Sciences and Research, Faridabad, Haryana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Priyanka Kardam, D – 81, Ground Floor, Saket, New Delhi - 110017, India.
E-mail: priyankakardam@gmail.com
Introduction
Collagen degradation is important both for carcinogenesis and in its progression. Research regarding the co-relation of collagen with Oral Epithelial Dysplasia (OED) and Oral Squamous Cell Carcinoma (OSCC) is less explored.
Aim
To elucidate the nature of collagen in Oral Epithelial Dysplasia (OED) and Oral Squamous Cell Carcinoma (OSCC) using Picrosirius Red Stain (PSR) under polarizing microscopy.
Materials and Methods
The study consisted of a total 40 samples which were divided into three groups. Group I included buccal mucosa as negative and irritation fibroma as positive control, group II consisted of OED and group III consisted of Oral Squamous Cell Carcinoma (OSCC). A histochemical analysis was conducted using PSR-polarization method by two independent observers.
Results
The control group shows predominantly reddish–orange birefringence. In OED with the advancement of grades, the colour changed from yellowish-orange colour to yellow-greenish with progressive increase in greenish hue. As OSCC regresses from well to poorly differentiated, the colour changed from reddish-orange to yellowish orange to greenish-yellow suggesting a transition from mature to immature collagen.
Conclusion
An observable gradual change in collagen of both OED and OSCC was noted as they were proceeding from benign to critical step. Thus, PSR is a useful tool for studying stromal changes as supporting collagen shows the transition in the form besides the alterations in epithelial cells.
Birefringence, Greenish yellow, Reddish orange, Yellowish orange
Introduction
Oral Potentially Malignant Disorders (OPMD) is defined by WHO in 2005 as the risk of malignancy being present in a lesion or condition either at time of initial diagnosis or at a future date [1]. These disorders are histopathologically reported as Oral Epithelial Dysplasia (OED) that is graded as mild, moderate or severe. The most commonly used grading system for OED by the histopathologists is the WHO criteria given in 1978 [2]. The severe form of OED is likely to transform to Oral Squamous Cell Carcinoma (OSCC) which is composed of two discrete compartments i.e. the malignant epithelial cells and the stroma in which they are dispersed [3]. During the development of OSCC, a number of changes occur in the epithelium including the basal lamina breakdown. Once the basal lamina is degraded, neoplastic epithelial cell interacts with the stroma, particularly the collagen and this interplay is a crucial determinant of tumour progression [4].
The collagenous tissue, “basic skeleton” of the stroma undergoes extensive changes during the evolution and progression of carcinoma. Firstly, it can have antagonistic effects on tumour progression either by inhibiting the host immune response or by resisting the tumour spread via inducing an abundant collagenous stroma (walling off effect) [5]. Secondly, proteolysis or change in the collagen composition can facilitate the mobilization of neoplastic cells into the stroma, therefore aiding subsequent invasion and metastasis [6].
Histochemically, to detect collagen fibers traditional stains such as Van Gieson and trichrome stains which are the combinations of two or more anionic dyes are routinely used. Since, these methods lack precise selectivity, hence they are not ideal for collagen detection in light microscopy [7]. Another disadvantage of these methods is that they fail to reveal very thin collagen fiber which can further lead to underestimation of collagen content [7,8].
The potential problems encountered using these traditional stains was first resolved by Constantine and Mowry in 1968 wherein a combination of Picrosirius Red (PSR) and polarization microscope was used for selective demonstration of collagen [9]. PSR stain is a combination of two anionic dyes i.e. Sirius red F3BA (Direct Red 80) dissolved in a saturated picric acid solution. Sirius Red is a hydrophilic dye which has sulphonic acid groups. These groups react with basic groups present in the collagen molecule [10]. There is a parallel arrangement of the dye molecules with the long axes of collagen fiber. This parallel relationship between dye and collagen results in an enhanced birefringence. This birefringent property results in bright yellow to orange colour of collagen when viewed under polarized light [11]. Further, various studies have been conducted using this combination and found that polarization colours of PSR stained collagen are not only due to fiber thickness but the packing of collagen molecules also plays a key role suggesting this method can be a useful tool for the structural analysis of collagen [12,13].
Numerous investigators have utilized this combination for the detection and analysis of collagen in oral submucous fibrosis or other oral pathologic conditions like odontogenic keratocyst or ameloblastic fibroma, etc. [14–17]. Ganganna K et al., correlated changes in birefringence of collagen fibers with degrees of epithelial dysplasia in OSF and observed that there was a gradual change in the polarization colours of thick collagen fibers observed in mild, moderate to severe epithelial dysplasia seen in the epithelium of OSMF [18]. Although, studies have been conducted on OSCC using this method, but to the best of our knowledge, there are no studies which have compared the grades of OSCC with that of OED using this method [19–21]. Thus, the present study was an attempt to elucidate the nature of collagen in Oral Epithelial Dysplasia (OED) and Oral Squamous Cell Carcinoma (OSCC) using Picrosirius Red Stain (PSR) under polarizing microscopy. The objective of the study was to examine the histochemical changes seen in collagen fibers in different grades of these lesions and to assess the relationship of this change with the nature of collagen with special reference to histopathological grading.
Materials and Methods
For the present study, an approval from the ethical committee of a tertiary referee university was taken prior and the details of patients were kept confidential. The study included a total of 40 diagnosed cases which were retrieved from the Department of Oral Pathology and Microbiology of Sudha Rustagi College of Dental Sciences and Research, Faridabad, Haryana, India. The diagnosis of the cases was based on the clinical and microscopic findings of the incisional biopsy. These 40 cases were divided into three groups [Table/Fig-1].
Total number of samples used in the present study
OED: Oral Epithelial Dysplasia; OSCC: Oral Squamous Cell Carcinoma
| GROUP | SPECIMEN | SAMPLE SIZE | TOTAL NUMBER |
|---|
| I –Control | Buccal mucosa- Negative control | 5 | 10 |
| Buccal mucosa irritation fibroma- Positive control | 5 | |
| II - Grades of OED | Mild dysplasia | 5 | 15 |
| Moderate dysplasia | 5 | |
| Severe dysplasia | 5 | |
| III-Grades of OSCC | Well differentiated OSCC | 5 | 15 |
| Moderate differentiated OSCC | 5 | |
| Poorly differentiated OSCC | 5 | |
From each paraffin block, two sections of 5-μm were obtained, one section was stained with Haematoxylin and Eosin (H&E) using standard protocol and other by the Picrosirius red stain. The procedure of PSR stain included the steps following deparaffinization and hydration in distilled water, the sections were then incubated in 0.1% (w/v) Direct Red 80/Sirius red (C.I- 365548- 5G, Sigma-Aldrich, Switzerland) with saturated Picric acid solution (Qualigens, Mumbai, India) for 1 hour at room temperature. This was followed by rinsing with distilled water, stained with Mayer’s haematoxylin (HI-media Labs. Mumbai, India) and differentiated in 1% HCl, alkalinization with tap water followed by the steps of dehydration and mounting.
Picrosirus red stained sections were examined under polarizing microscopy (Olympus BX41 TF) at a magnification of x100 for collagen fiber in the connective tissue and differences in the polarizing colours of the collagen fibers in different lesions were analysed. In OSCC, collagen fibers around tumour islands or cords were considered and areas showing dense inflammation or epithelial ulceration can have an impact on collagen arrangement thus were excluded. All the samples were analysed by two observers to check inter-observer variability and the data was computed.
Results
The H&E stained slides were observed under normal light and it was noted that none of the groups showed excessive collagen fiber deposition except the positive control i.e., buccal mucosa irritation fibroma. Subsequently, PSR stained slides were examined under polarizing light for collagen fiber arrangement. Group I showed predominantly reddish-orange birefringence [Table/Fig-2,3] and group II exhibited a gradual change in the polarizing colours from yellowish-orange to slight increase in greenish tinge finally to intensified greenish hue on progressing from mild to severe OED [Table/Fig-2,4]. However, in Group III, a gradual change in the polarizing colours from reddish orange to yellowish orange to greenish yellow was seen from well differentiated to poorly differentiated OSCC [Table/Fig-2,5]. Inter-observer agreement was analysed using Kappa statistics and 91% agreement was found between the two observers for collagen birefringence.
Showing the polarizing colours observed (x100) in each of 40 samples
| GROUP | SAMPLE | COLOUR OBSERVED by Observer 1 (x100) | COLOUR OBSERVED BY OBSERVER 2 (x100) |
|---|
| I. Control | Buccal mucosa- Negative control | Predominantly reddish-orange | Predominantly reddish |
| Buccal mucosa irritation fibroma- Positive control | Predominantly reddish-orange | Predominantly reddish-orange |
| II. OED | Mild dysplasia | Yellowish orange with minimal areas of red birefringence | Yellowish orange |
| Moderate dysplasia | Yellowish orange colour with slight greenish hue | Yellowish orange colour with slight greenish hue |
| Severe dysplasia | Intensified Greenish hue | Greenish hue |
| III. OSCC | Well differentiated OSCC | Reddish orange birefringence | Reddish orange birefringence |
| Moderately differentiated OSCC | Yellowish orange birefringence | Yellowish orange birefringence to slight greenish hue |
| Poorly differentiated OSCC | Greenish yellow hue | Greenish-yellow hue |
OED: Oral Epithelial Dysplasia; OSCC: Oral Squamous Cell Carcinoma
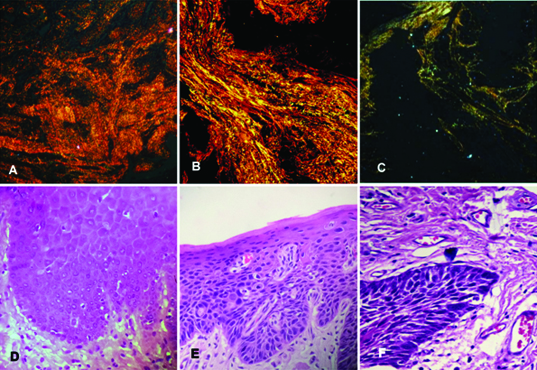
a) Photomicrograph of histologically normal buccal mucosa as negative control showing predominantly reddish-orange birefringence. (Picrosirius red stain, X100)
b) Photomicrograph of buccal mucosa irritation fibroma as positive control showing reddish-orange colour. (Picrosirius red stain, X100)
c) Photomicrograph of histologically normal buccal mucosa used as negative control (H&E stain, X100)
d) Photomicrograph of buccal mucosa irritation fibroma used as positive control (H&E stain, X100)
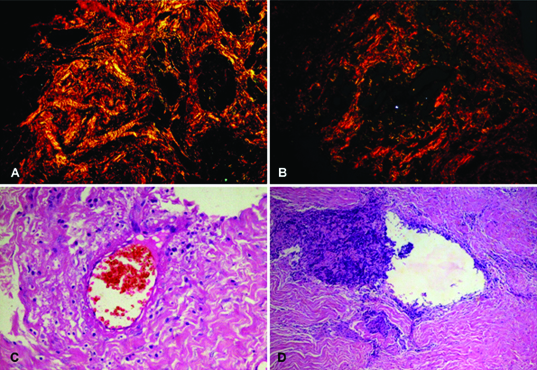
a) Photomicrograph of Mild dysplasia showing predominantly yellowish orange colour with minimal areas of red birefringence. (Picrosirius red stain, X 100)
b) Photomicrograph of Moderate dysplasia showing yellowish orange colour with greenish hue. (Picrosirius red stain, X 100)
c) Photomicrograph of Severe dysplasia showing intense greenish hue. (Picrosirius red stain, X100)
d) Photomicrograph of Mild dysplasia seen under a light microscope (H&E stain, X100)
e) Photomicrograph of Moderate dysplasia seen under a light microscope (H&E stain, X100)
f) Photomicrograph of Severe dysplasia seen under a light microscope (H&E stain, X100)
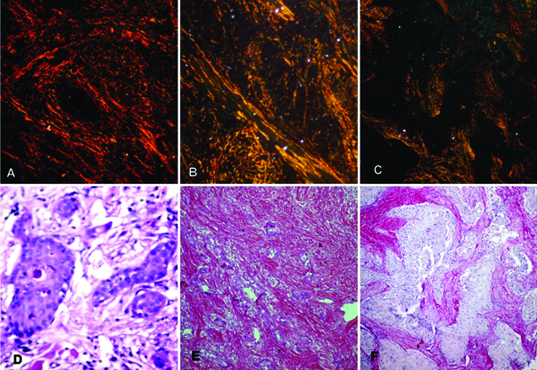
a) Photomicrograph of Well differentiated OSCC showing reddish orange birefringence. (Picrosirius red stain, X100)
b) Photomicrograph of Moderately differentiated OSCC showing yellowish orange with sight greenish hue. (Picrosirius red stain, X100)
c) Photomicrograph of Poorly differentiated OSCC showing greenish yellow colour. (Picrosirius red stain, X100)
d) Photomicrograph of Well differentiated OSCC seen under a light microscope (H&E stain, X100)
e) Photomicrograph of Moderately differentiated OSCC seen under a light microscope (H&E stain, X100)
f) Photomicrograph of Poorly differentiated OSCC seen under a light microscope (H&E stain, X100)
Discussion
The development of OSCC is believed to occur in a stepwise fashion, beginning with OED progressing to carcinoma in situ and finally to OSCC. An essential step for OED to transform into OSCC is to invade the stroma by breakdown of basement membrane [22]. In general, the development of OSCC is intrinsically correlated with the surrounding stroma because during carcinogenesis, the tumour requires its stroma to grow beyond 1-2 mm size. The stroma acts as a mixed blessing, it provides the environment for nourishment, exchange of gas and waste material and also restricts the influx of inflammatory cells for the neoplastic cells. On the other hand, neoplastic cells in stroma may either induce desmoplasia or cause the lysis [20].
Thus, besides the malignant epithelial cells, the supporting stroma is equally important component for the existence and progression of OSCC. Collagen, one of the major elements of the stroma, is primarily affected in the stromal changes at the site of tumour cell invasion. Although numerous studies have been conducted on the relationship of stromal reaction with OSCC but research regarding the early stromal reaction in oral potentially malignant disorders is fragmentary [23]. Thus, the present study assessed the collagen fiber changes with progressive grades of OED and OSCC. In OED, beginning from mild with increasing grades of dysplasia, the birefringence alters from yellowish-orange to finally greenish colour passing through slight greenish hue and similar findings were mentioned by Yokoyama M [24]. In different grades of OSCC, there is a gradual change in birefringence ranges from reddish orange to yellowish orange and eventually to yellowish green. Such changes were in accordance with the study done by Aparna V et al., Kalele KK et al., and Manjunatha BS et al., [19–21]. However, another study done by Martins GB et al., suggested some changes in the collagen but did not mention colour gradation as the grades were progressive [25].
It is well-known that the PSR is considered to be one of the most widely used stain to visualize collagen fibers in histological tissues. For reasons like it’s less tendency to fade unlike trichrome stain and has more selectivity thus making it ideal and superior for both staining and quantification of collagen. The reason behind its enormous application is that PSR enhances the birefringence properties of collagen which give rise to variety of colours [13].
Firstly, Junquiera et al., and Szendroi et al., suggested that the spectrum of colours may be due to the fiber thickness and they described that thin fibers usually show green to yellowish hue whereas thick fibers show orange to red polarization colour [26,27]. Later on, Dayan et al., proposed that packing of these collagen fibers also plays an important role in the pattern of polarization colours of PSR stained collagen. Tightly packed and well aligned collagen fibers showed polarization colours of longer wavelengths (reddish orange) [28]. Even, Trau H et al., said that PSR stained sections by polarizing microscopy can serve as a procedure for differentiating procollagens, intermediates and other non-tightly packed collagen fibers from normal tightly packed fiber [29]. Sharf Y et al., conducted a study using nuclear resonance technique which revealed a colour profile of orange to red which corresponded to the well packed fibers and the green to greenish yellow to poorly packed fibers [30]. Recently, Rich L and Whittaker P demonstrated that PSR with polarization microscopy is the best method to analyse structure of collagen [13]. Thus, it can be stated that primarily the compactness of collagen can be studied by the change in polarization colour.
In the present study, the colour detected in early stages of OSCC (i.e. in well differentiated grade) was reddish-orange with advancing grade the colour changed to yellowish-green. A similar progressive change was noted in the OED with varying grades. Hence, the present study indicated that the change in the polarizing colour of collagen fiber which coincided with the change found in the stages of both OED and OSCC. However, this peculiar change in colour with respect to the type of collagen fibers as the lesion is invading and advancing exist but the exact mechanism behind such process is still unclear.
It is a well-known fact, that the hallmark of carcinoma is the neoplastic cell migration and invasion. During the transformation from dysplasia to carcinoma, hypoxia arises which induces genetic instability and accelerate angiogenesis thus making the stroma edematous and unstable. As carcinoma progresses, neoplastic cell transform collagen mainly by the production of Carcinoma-Associated Fibroblasts (CAFs) and increase collagenolytic enzyme activity. This altered fibroblast phenotype contribute to the production of altered collagen. Also, by increased formation of collagenases, the invading neopalstic cell is able to dissolve the collagen eventually leading to disarranged stroma [31]. Several studies have already proven that during tumourigenesis different types of collagen, in particular collagen type I (thick) is synthesized which when stained with PSR gives reddish orange hue [27]. The severe grades of both OEDD and SCC showed yellowish-greenish hue suggesting the presence of type III (thin) collagen fibers. Thus, our findings confirm previous results that collagen type I is decreased and replaced by type III during the transformation of initial to higher grades, both in OED and in OSCC. Therefore, suggesting that as the cancer progresses, the surrounding stroma co-evolves into the active state through continuous tumour-stroma interactions [6,20,24,25].
So far all the PSR stain related studies have used linear polarized light, though, there are some limitations using this technique. The first disadvantage of using linear polarized light is that PSR-stained fibers will appear dark if they are aligned parallel to the transmission axis of either of the two linearly polarizing filters. This can be overcome by using rotating microscope stage which will change the orientation of the tissue section with respect to the transmission axes. But some collagen fibers are frequently crimped or wavy and so will appear dark irrespective of rotated microscope stage. Thus, the total collagen content especially in tissue containing large amounts of wavy fibers may be underestimated. Here, we should emphasize that the fiber hue does not permit identification of collagen fiber type as some have suggested. Secondly, type III fibers are usually thinner than type I fibers but the green colour does not necessarily signify type III and can also represent either an immature type I or sectioning artifact smeared of thick type I fiber. Thirdly, materials such as keratin and fibrin are weakly birefringent which is almost similar to that of the thinnest collagen fibers and thus complicating the analysis [11].
Conclusion
In the present study, observable collagenous changes were seen in progressive grades of OED and OSCC. We have demonstrated that the combination of PSR with polarization microscopy is a powerful tool for its structural analysis and the colour changes observed in collagen fibers reflect its gradual shift from thicker towards thinner fibers in stroma during tumour progression. Like any other technique even PSR with polarization microscopy has some limitations. Thus, it should be supplemented with gold standard i.e., H&E and molecular markers on a larger sample including the tumour invading front to extrapolate this knowledge.
OED: Oral Epithelial Dysplasia; OSCC: Oral Squamous Cell Carcinoma
[1]. George A, Potentially malignant disorders of oral cavityOral Maxillofacial Pathology Journal 2011 2(1):95-100. [Google Scholar]
[2]. Kardam P, Rehani S, Mehendiratta M, Sahay K, Mathias Y, Journey of leukoplakia so far – an insight on shortcomings of definitions and classificationsJ Dent Oral Disord Ther 2105 3(2):1-6. [Google Scholar]
[3]. Dvorak HF, Tumours: wounds that do not heal: Similarities between tumour stroma generation and wound healingN Engl J Med 1986 315(26):1650-59. [Google Scholar]
[4]. Yokoyama M, Alterations in stromal reaction during tumour progression in oral mucosaJ Hard Tissue Biology 2011 20(1):23-30. [Google Scholar]
[5]. Ritchie AC, Boyd’s Textbook of Pathology 1990 PhiladelphiaLea & Febiger:266 [Google Scholar]
[6]. Fuentes B, Duaso J, Droguett D, Progressive extracellular matrix disorganization in chemically induced murine oral squamous cell carcinomaISRN Pathology 2012 [Google Scholar]
[7]. Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, Rapid quantification of myocardial fibrosis: a new macro-based automated analysisCell Oncol (Dordr) 2011 34(4):343-44. [Google Scholar]
[8]. Whittaker P, Kloner RA, Boughner DR, Pickering JG, Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized lightBasic Res Cardiol 1994 89(5):397-410. [Google Scholar]
[9]. Constantine VS, Mowry RW, Selective staining of human dermal collagen II. The use of picrosirius red F3BA with polarization microscopyJ Invest Dermatol 1968 50(5):419-23. [Google Scholar]
[10]. Junqueira LC, Bignolas G, Brentani RR, Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sectionsHistochem J 1979 11(4):447-55. [Google Scholar]
[11]. Montes GS, Junqueira LC, The use of the picrosirus-polarization method for the study of the biopathology of collagenMem Inst Oswaldo Cruz 1991 86:1-11. [Google Scholar]
[12]. Rabau MY, Dayan D, Polarization microscopy of picrosirius red stained sections: A useful method for qualitative evaluation of intestinal wall collagenHistol Histopathol 1994 9(3):525-28. [Google Scholar]
[13]. Rich L, Whittaker P, Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distributionBraz J Morphol Sci 2005 22(2):97-104. [Google Scholar]
[14]. Rajalalitha P, Vali S, Molecular pathogenesis of oral submucous fibrosis - a collagen metabolic disorderJ Oral Pathol Med 2005 34(6):321-28. [Google Scholar]
[15]. Hirshberg A, Sherman S, Buchner A, Dayan D, Collagen fibers in the wall of odontogenic keratocysts: A study with picrosirius red and polarizing microscopyJ Oral Pathol Med 1999 28(9):410-12. [Google Scholar]
[16]. Nyska A, Dayan D, Ameloblastic Fibroma in a young catJ Oral Pathol Med 1995 24(5):233-36. [Google Scholar]
[17]. Ashalata G, Baghirath PV, Krishna AB, Kumar PU, Tom A, Quantitative and qualitative analysis of collagen in oral submucous fibrosisJ NTR Univ Health Sci 2012 1:99-105. [Google Scholar]
[18]. Ganganna K, Shetty P, Shroff SE, Collagen in histologic stages of oral submucous fibrosis: A polarizing microscopic studyJ Oral Maxillofac Pathol 2012 16(2):162-66. [Google Scholar]
[19]. Kalele KK, Managoli NA, Roopa NM, Kulkarni M, Bagul N, Kheur S, Assessment of collagen fiber nature, spatial distribution, hue and its correlation with invasion and metastasis in oral squamous cell carcinoma and surgical margins using Picro Sirius red and polarized microscopeJ Dent Res Rev 2014 1:14-17. [Google Scholar]
[20]. Venigella A, Charu S, Evaluation of collagen in different grades of oral squamous cell carcinoma by using the picrosirius red stain -Histochemical studyJ Clin Diagn Res 2010 4:3444-49. [Google Scholar]
[21]. Manjunatha BS, Agrawal A, Shah V, Histopathological evaluation of collagen fibers using picrosirius red stain and polarizing microscopy in oral squamous cell carcinomaJ Can Res Ther 2015 11(2):272-76. [Google Scholar]
[22]. Silverman S, American Cancer SocietyOral cancer 1998 LondonB.C. Decker Inc:4 [Google Scholar]
[23]. Koontongkaew S, The tumour microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomasJ Cancer 2013 4(1):66-83. [Google Scholar]
[24]. Yokoyama M, Alterations in stromal reaction during tumour progression in oral mucosaJ Hard Tissue Biology 2011 20(1):23-30. [Google Scholar]
[25]. Martins GB, Reis SR, Silva TM, Collagen type I expression in squamous cell carcinoma of the oral cavityPesqui Odontol Bras 2003 17(1):82-88. [Google Scholar]
[26]. Junqueira LCU, Montes GS, Sanchez EM, The influence of tissue section thickness on the study of collagen by the picrosirius polarization methodHistochemistry 1982 74:153-56. [Google Scholar]
[27]. Szendroi M, Vajta G, Kovacs L, Schaff Z, Lapis K, Polarization colours of collgaen fibers: A sign of collagen production activity in fibrotic processActa Morphol Hung 1984 32:47-55. [Google Scholar]
[28]. Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M, Are the polarization colours of picrosirius red-strained collagen determined only by the diameter of the fibers?Histochemistry 1989 93:27-29. [Google Scholar]
[29]. Trau H, Dayan D, Hirshberg A, Connective tissue nevi collagens. Study with picrosirius red and polarizing microscopyAm J Dermatopathol 1991 13:374-77. [Google Scholar]
[30]. Sharf Y, Knubovets T, Dayan D, The source of the NMR detected motional anisotropy of water in blood vessel wallsBiophys J 1997 73:1198-204. [Google Scholar]
[31]. Daley WP, Peters SB, Larsen M, Extracellular matrix dynamics in development and regenerative medicineJ Cell Sci 2008 121(3):255-64. [Google Scholar]