Fluoride which has been described as an essential nutrient is extremely useful for the normal development and growth of human beings [1]. As per WHO, 0.6 ppm fluoride ingestion is useful, when fluoride gets accumulated in hard tissues of the body it plays an important role in mineralization of bone and teeth [2].
At high levels it causes dental and skeletal fluorosis. Dental fluorosis occurs with exposure of more than 1-1.5 ppm, in developing enamel which is characterized mainly by mottling of the teeth [3]. The adverse effects of high fluoride intake are also observed in soft tissues which are known to affect collagen synthesis, inhibit enzymes such as those involved in the pentose pathway, antioxidant defense system and the myosin ATPase pathway [4]. In the present study, we investigated the effects of different concentrations of sodium fluoride (NaF) in order to evaluate cytomorphology of exfoliated oral mucosal cells in Albino rats. The volume of nucleus and cell were assessed using image analysis with multivariant microscopic parameters which reflect the N/C ratio, density i.e. overall cellular metabolic activity.
Materials and Methods
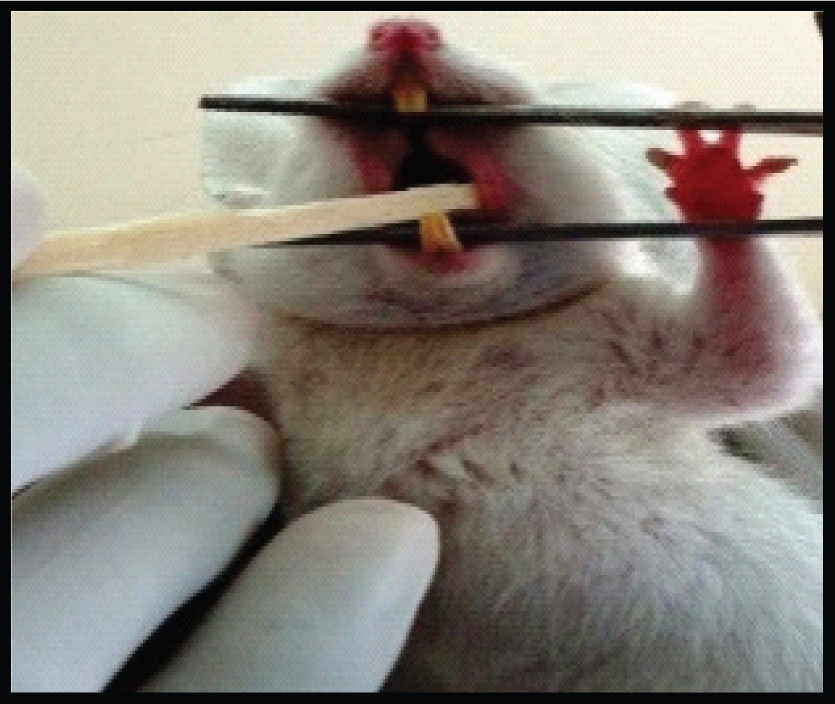
The study was designed in the year 2013 to evaluate and to compare the cytomorphology of exfoliated oral mucosal cells of Albino Wistar rats among various concentrations of fluoride for a time period of 42 days. This study was conducted in the Department of Oral and Maxillofacial Pathology, Kamineni Institute of Dental Sciences, Narketpally, Nalgonda district which was approved by the Institutional Animal Ethical Committee which was registered as KIDS/IEC/ORALPATH/11-10. Male Albino Wistar rats weighing 80-120 gram were used in the experiments and housed in polycarbonated cages with compressed fiber bedding. Commercial pellet diet and water were provided. All the rats were free from any concomitant systemic diseases. The rats were fed for a time period of 42 days as the turnover rate of rats oral mucosal cells was around 32-52 days. The sampling technique was that the animals were divided into control group and study group, four animals each group. The control group was fed with 1ppm concentration of NaF mixed with distilled water, for a period of 42 days. The study group was divided into three sub groups as A group which were fed with 25 ppm of NaF mixed with distilled water, B group which was fed with 50 ppm of NaF mixed with distilled water, C group which was fed with 100 ppm concentration of NaF mixed with distilled water, for a period of 42 days. With a sterilized, moistened wooden spatula the buccal mucosa was gently scraped [Table/Fig-1]. The cells were immediately smeared on precleaned microscopic slides and fixed immediately by suspension in absolute alcohol. The smears which are fixed with absolute alcohol are subjected to PAP staining procedure.
Buccal Mucosa smears taken with wooden spatula
After preparation of the slides, 20 largest cells in slide were selected & the sections were subjected to morphometric analysis to measure nuclear followed by cellular changes to avoid overlapping using Image Pro Insight, version 8 (Media cybernetics, USA), and were captured using a CCD camera attached to a binocular research microscope with a 40x objective, with the final image captured on the monitor with a magnification of 1000x. Microscopic fields were selected randomly, commencing with first field on the left hand side of the section, and then moving the stage to the next field, to include a minimum of 4-5 fields from each section. All the measurements were classified and saved in Microsoft excel for further statistical analysis.
For the assessment of the differences between the cases and the controls, six parameters were selected, which were the following:
Maximum diameter of the nucleus
Minimum diameter of the nucleus
Perimeter of the nucleus
Maximum diameter of the cell
Minimum diameter of the cell
Perimeter of the cell.
Statistical Analysis
SPSS version 19 was used for statistical analysis. The significance of the results obtained from the control and study groups were statistically analysed by one-way ANOVA test. Multiple comparisons between the groups were assessed for statistical significance using Tukey Kramer Honestly Significant Difference (HSD) test. The p-value < 0.05 was considered to be statistically significant. Mean and standard deviation of cases and controls was also determined.
Results
The results of the maximum diameter of the nucleus showed that p-value was statistically significant (p-value: 0.000 & p-value: 0.001) [Table/Fig-2]. The minimum diameter of the nucleus showed that p-value was statistically not significant (p-value: 0.198) [Table/Fig-3]. The perimeter of the nucleus showed that p-value was statistically significant (p-value: 0.001) [Table/Fig-4]. The results of maximum, minimum diameter and perimeter of the cell showed that p-value was statistically not significant (p-value: 0.791, 0.600 & 0.719) [Table/Fig-5,6 and 7].
Maximum diameter of nucleus
| Sum of Squares | Df | Mean Square | f | Sig. |
|---|
| Max.N | Between Groups | 185.779 | 4 | 46.445 | 6. 002 | .000 |
| Within Groups | 056.355 | 395 | 7.738 | | |
| Total | 3242.134 | 399 | |
Minimum diameter of nucleus
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Min.N | Between Groups | 29.620 | 4 | 7.405 | 1.512 | .198 |
| Within Groups | 1933.986 | 395 | 4.896 | | |
| Total | 1963.606 | 399 | |
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| P.N | Between Groups | 1177.450 | 4 | 294.363 | 5.024 | .001 |
| Within Groups | 23143.568 | 395 | 58.591 | | |
| Total | 24321.018 | 399 | |
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Max.C | Between Groups | 164.987 | 4 | 41.247 | .424 | .791 |
| Within Groups | 38441.794 | 395 | 97.321 | | |
| Total | 38606.781 | 399 | |
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| Min C | Between Groups | 126.657 | 4 | 31.664 | .689 | .600 |
| Within Groups | 18140.699 | 395 | 45.926 | | |
| Total | 18267.356 | 399 | |
| Sum of Squares | df | Mean Square | F | Sig. |
|---|
| P.C | Between Groups | 1418.248 | 4 | 354.562 | .524 | .719 |
| Within Groups | 267524.207 | 395 | 677.276 | | |
| Total | 268942.455 | 399 | |
Multi parametric TUKEY HSD test results indicated that p-value was found to be statistically significant for maximum diameter of the nucleus and perimeter of the nucleus but statistically not significant for minimum diameter of nucleus between controls and the categorized groups (A group, B group, C group) and within the categorized groups. In case of maximum diameter of the cell, minimum diameter of the cell and perimeter of the cell the results indicate that p-value was found to be statistically not significant between controls and the categorized groups (A group, B group, C group) and within the categorized groups.
Discussion
Fluoride exists as the fluoride ion or as hydrofluoric acid in the body fluids [5]. Chronic poisoning from long term exposure is a serious health problem in many parts of the world where drinking water contains more than 1- 1.5 ppm of fluoride [3]. Moderate amounts lead to dental defects, but long term ingestion of large amounts can lead to potentially severe skeletal problems [6]. In human beings fluorosis is mainly caused by drinking fluorinated water, burning coal and drinking tea [7]. Osseous & soft tissues are damaged due to fluoride intoxication [8,9]. Effects of fluorosis can be skeletal or non skeletal [10]. Mucosal abnormalities are common with skeletal fluorosis [11]. Higher concentrations of fluoride are known to affect collagen synthesis, inhibit enzymes involved in the pentose pathway, antioxidant defense system and themyosin ATPase pathway. Furthermore fluoride is also known to cross the cell membrane and enter soft tissues [4].
Exfoliative cytology is the microscopic examination of shed or desquamated cells from the epithelial surface usually the mucous membrane, including collection of scrapings of tissue surface or body fluids such as sputum, saliva etc, [12]. Exfoliated cells can be demonstrated with special procedures like Immunohistochemistry (IHC), image analysis (Image Pro Insight, version 8) which help in diagnosing diseases [13].
Our present study was to evaluate different concentrations of fluoride on oral mucosal cells of Albino Wistar rats. To the best of our knowledge, this is the first study of its kind to test nuclear and cellular parameters of oral mucosal alterations with the help of exfoliative cytology in fluoride induced Albino Wistar rats.
The selected 16 rats were divided into four groups with four rats in each group. Different groups were fed with different concentrations (1ppm, 25 ppm, 50 ppm & 100 ppm) of sodium fluoride which was mixed with distilled water everyday freshly, over a period of 42 days. Samples were collected after 42 days from oral buccal mucosa of the rats using exfoliative cytology. The following parameters – maximum & minimum diameter of the nucleus and the cell, were used to assess the changes in the volume of the nucleus and the cell respectively. Perimeter of the nucleus and the cell were used to assess the changes in surface area of the nucleus and the cell. All these parameters reflect the activity of the cell. PAP staining procedure was used for staining the prepared buccal smears and analysis of smears were made by image analysis software.
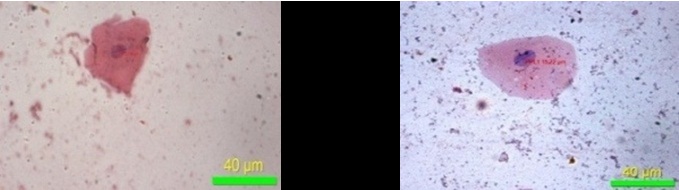
The present study has demonstrated that, there was decrease in the mean values of maximum diameter [Table/Fig-8], minimum diameter of the cell [Table/Fig-9] and increase in the mean values of maximum diameter [Table/Fig-10], minimum diameter of the nucleus [Table/Fig-11] in fluoride induced study groups when compared to controls. Mean value of perimeter of the cell also decreased [Table/Fig-12] with increase in the perimeter of nucleus [Table/Fig-13]. Various studies have been conducted to know the effects of fluoride on various human organs, but very few have highlighted its effect on oral mucosa. He LF et al., had done a study on ‘DNA damage, apoptosis and cell cycle changes induced by fluoride in rat oral mucosal cells and hepatocytes’ & noted changes in cell morphology [14]. Madhusudan et al., showed that fluorosis induces oxidative stress, DNA damage and apoptosis which can be the reasons for increase in nuclear size and decrease in cell size [15]. Bhussry BR et al., elaborated lethal action of sodium fluoride involving two mechanisms: one causing death in few hours & other in 3-10 days [16]. Zhang Y et al., showed that excess fluoride affects cell activity, retarded cell cycle at S phase & induced apoptosis [17]. The present study correlates with the observations made by the above authors.
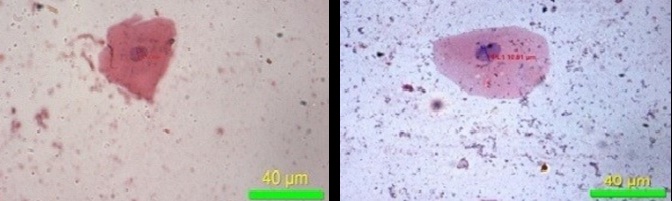
Maximum diameter of cell (40X)
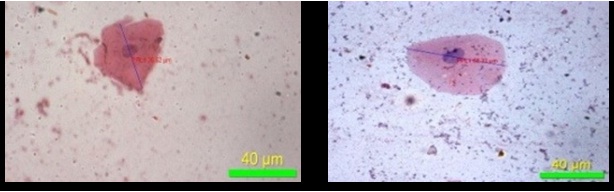
Minimum diameter of cell (40X)
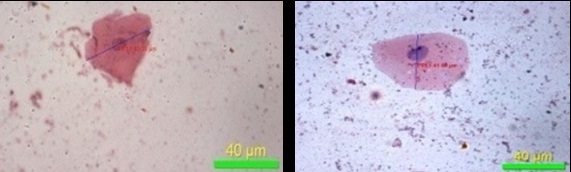
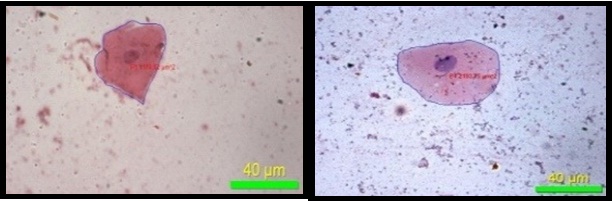
Maximum diameter of nucleus (40X)
Minimum diameter of nucleus (40X)
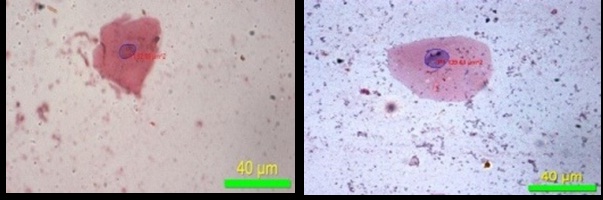
Perimeter of nucleus (40X)
The causes of cell injury range from oxygen deprivation, physical agents, chemical agents and drugs, infectious agents, immunologic reactions, genetic derangements and nutritional imbalances. The cellular response to injurious stimuli depends on the nature of the injury, its duration, and its severity. Small doses of a chemical toxin may induce reversible injury, whereas large doses of the same toxin might result either in instantaneous cell death or in slow, irreversible injury leading in time to cell death. The consequences of cell injury depend on the type, state, and adaptability of the injured cell. The cell’s nutritional and hormonal status and its metabolic needs are important in its response to injury. The cellular components that are most frequently damaged by injurious stimuli include mitochondria, cell membranes, the machinery of protein synthesis and the DNA in nuclei [18].
Due to its double edge nature acute or chronic exposure to high fluoride doses results in adverse health effects. The molecular mechanisms underlying fluoride toxicity are different by nature [19]. Excess fluoride induces oxidative stress, DNA damage, apoptosis and cell cycle changes in rat oral mucosal cells and hepatocytes [14,15]. These mechanisms are described individually below. The following are three hypotheses that describe various molecular mechanisms of apoptosis due to excess fluoride [18].
1. Apoptosis due to influx of calcium and loss of calcium homeostasis: Increased intracellular Ca2+ causes cell injury by several mechanisms resulting in opening of the mitochondrial permeability transition pore, or activates a number of enzymes, with potentially deleterious cellular effects which include phospholipases (which cause membrane damage), proteases (which break down both membrane and cytoskeletal proteins), endonucleases (which are responsible for DNA and chromatin fragmentation), and ATPases (thereby hastening ATP depletion) or may also result in the induction of apoptosis, by direct activation of Caspases [18].
2. Apoptosis due to depletion of ATP: Glycolysis is a major pathway for ATP pathway synthesis in tissues [20]. Excess fluoride inhibits enzyme enolase which is designed to maintain the cell’s energy sources by generating ATP through metabolism of glucose derived from glycogen, which is essential for conversion of glucose into lactic acid in glycolysis. This leads to decreased energy production in the cell causing cellular changes like cellular condensation and nuclear changes like nuclear fragmentation [18].
3. Apoptosis due to mitochondrial damage: Several proteins like cytochrome c and proteins that indirectly activate apoptosis inducing enzymes called caspases increase permeability of the outer mitochondrial membrane resulting in leakage of these proteins into the cytosol, and death by apoptosis. Persistent or excessive injury, however, causes cellular changes like cellular condensation and nuclear changes like nuclear fragmentation. These biological changes lead to decrease in the cell size and increase in the nucleus size thus causing apoptosis [18].
Conclusion
Research on effects of fluoride on skeletal and dental tissue was done extensively, but it is of utmost important to gain more knowledge of its effects on oral soft tissues. Hence this study was aimed to assess fluoride effects on oral mucosa in Albino wistar rats. The observations of our present study revealed that cellular changes occur with severity of fluoride and also showed dysplastic alterations caused by it. But more studies have to be done extensively for longer duration of time with a larger sample in humans to confirm these alterations and also to assess the long term effects caused by fluoride an oral mucosa in order to elucidate their mechanism.