Colorectal carcinoma (CRC) is one of the leading cancers in the developed countries & its incidence varies considerably throughout the world as environmental, dietary and genetic factors play key roles in its aetiology. Even with advances in the treatment of CRC, it remains the second leading cause of death worldwide next to lung cancer. A number of prognostic factors have been studied; evaluated and targeted therapy has been developed. One such prognostic factor is neuroendocrine differentiation in CRC [1–3]. Neuroendocrine differentiation can be encountered in many human neoplasms derived from different organs and systems which can be detected using immunohistochemistry and ultrastructural techniques [4]. There are studies [5–19] done on this aspect which have showed conflicting results ranging from worse prognosis to no prognostic significance whatsoever. Neuroendocrine differentiation in CRC is detected by demonstrating expression of neuroendocrine markers such as chromogranin A (Chg A), synaptophysin (Syn) and neuron specific enolase (NSE) by immunohistochemical methods for tumour differentiation [5].
This study was conducted to determine the prognostic significance of neuroendocrine differentiation in moderately, poorly and undifferentiated CRC using immunohistochemical stains such as Chg A and Syn.
Materials and Methods
The study comprised of a retrospective analysis of 84 cases of resected specimens of CRCs that was studied over a period of 3 years from January 2009 to December 2011 in the Department of Pathology of a tertiary health care hospital in Mangalore. Colonoscopic biopsy specimens were excluded from the study. The clinical history & endoscopic findings were retrieved from medical archives. Macroscopic findings were recorded and the haematoxylin & eosin stained slides were reviewed. The histopathological type, grade and stage of the tumour were analysed. The CRCs were graded based on degree of gland formation into well differentiated (G1), moderately differentiated (G2), poorly differentiated (G3) & undifferentiated (G4) as given in [Table/Fig-1].
Criteria for the grading of colorectal carcinoma [2]
| Grade | Descriptivenomenclature | Criteria | AJCCRecommendations |
|---|
| G1 | Well differentiated(WD) | >95% gland formation Majority(>75%) of glands are smooth andregular. No significant componentof high grade nuclei | Low grade |
| G2 | Moderatelydifferentiated (MD) | 50-95% gland formation | Low grade |
| G3 | Poorlydifferentiated (PD) | <50% gland formation | High grade |
| G4 | Undifferentiated | No apparent gland formation | High grade |
Further, Immunohistochemical staining was performed to determine neuroendocrine differentiation using Chg A & Syn only on 53 cases of moderately differentiated (G2), poorly differentiated (G3) & Undifferentiated (G4) adenocarcinomas. The remaining 31 cases of well differentiated CRCs (G1) were not subjected to immunohistochemical staining.
Intensity of staining was categorized as grade 0 (no expression), grade 1(< 2% cells staining positive), grade 2 (2-10% cells staining positive), grade 3 (10-30% cells staining positive) & grade 4 (>30% cells staining positive) [6–9,11,13,15]. Follow-up data of the cases were recorded.
Statistical Analysis
The survival analysis was performed using Kaplan Meier graph. Various clinical & morphological features were analysed for their frequency and were compared with the final diagnosis and survival using cross tabs and Chi-square value (x2) with one degree freedom, wherever appropriate. In the present study, a p value of <0.05 was considered significant for the performed tests. All tabulations and statistical analysis was done using IBM SPSS 20 data software.
Results
Colorectal carcinomas with neuroendocrine differentiation did not show any characteristic neuroendocrine morphology such as rosette formation, organized nests, cords, trabeculae or ribbons. However, a total of 18 out of the 53 cases (33.9%) exhibited neuroendocrine differentiation, which included 11 cases of moderately differentiated CRC (61%), 4 cases of mucinous CRC (22%), 2 cases of poorly differentiated CRC (11%) and a case of Signet ring CRC (6%). The patients age among CRCs with neuroendocrine differentiation ranged from 28-76 years (Mean 54±14.5 years). Among them, 11 were males and 7 were females, with the M:F ratio being 1.6:1. There was no predilection to site (Right colon: Left colon:: 1.1:1). Rectosigmoid was the most common site of involvement among CRC with neuroendocrine differentiation (7 cases, 39%).
The intensity of neuroendocrine differentiation were grade 0 in 58.5%, grade 1 in 7.5%, grade 2 in 9.4%, grade 3 in 13.2% & grade 4 in 11.3% of cases [Table/Fig-2,3]. There was no significant correlation of histopathological subtype with neuroendocrine differentiation (p=0.6).
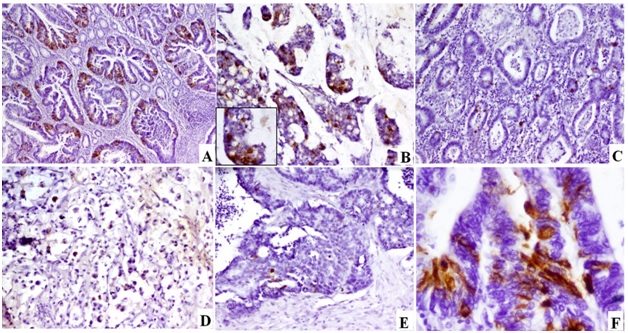
Intensity of Chromogranin A staining (Chg A)
a) MD CRC showing Chg A positivity in >30% of the tumour tissue [Chg A Immunoperoxidase, 40x]; b) Mucinous CRC showing Chg A positivity in 10-30% of the tumour tissue [Chg A Immunoperoxidase,100x] Inset: High power view of one of the tumour nests [Chg A Immunoperoxidase, 400x]; c) MD CRC showing Chg A positivity in >2% of the tumour tissue [Chg A Immunoperoxidase,40x]; d) SR CRC showing Chg A positivity in 2-10% of tumour tissue [Chg A Immunoperoxidase,100x]; e) PD CRC showing Chg A positivity in <2% of the tumour tissue [Chg Immunoperoxidase,40x]; f) High power view showing granular cytoplasmic positivity for Chg A [Chg A Immunoperoxidase, 400x]
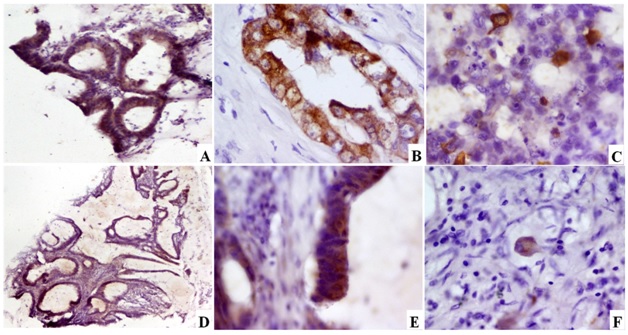
Intensity of Synaptophysin staining (Syn)
a) MD CRC showing Syn positivity in >30% of the tumour tissue [Syn Immunoperoxidase,100x]; b) MD CRC showing Syn positivity in 10-30% of the tumour tissue [Syn Immunoperoxidase, 400x]; c) PD CRC showing Syn positivity in 2-100% of the tumour tissue [Syn Immunoperoxidase,400x]; d) Mucinous carcinoma showing Syn positivity in 10-30% of the tumour tissue [Syn Immunoperoxidase, 40x]; e) High power view showing granular cytoplasmic positivity for Syn [Syn Immunoperoxidase, 400x]; f) SR CRC showing granular cytoplasmic positivity [Syn Immunoperoxidase, 400x]
Of the 18 cases of CRCs showing neuroendocrine differentiation, most of the cases belonged to Grade II (61%) & Stage II & III (83%). Similarly, there was no significant correlation with the grade and stage of the tumour with neuroendocrine differentiation (p=0.1, p=0.4). The correlation of the clinicopathological findings and neuroendocrine differentiation is given in [Table/Fig-4].
Correlation between clinicopathological findings and neuroendocrine differentiation of CRCs
| Variables | Colorectal Carcinoma | p value |
|---|
| Non NE groupn=35 | NE groupn=18 |
|---|
| Sex |
| M | 22 | 11 | 0.6 |
| F | 13 | 7 |
| Age |
| Mean ±SD (years) | 58.9 ±13.7 | 53.94 ± 15.63 | 0.7 |
| Range | 30 to 87 years | 28 to 76 years |
| Location |
| C/A/T | 6/5/4 | 5/1/2 | 0.2 |
| D/S | 1/9 | 0/3 |
| R | 8 | 4 |
| NS | 2 | 3 |
| Gross |
| Polypoidal | 9 | 3 | 0.9 |
| Thickening | 5 | 3 |
| Ulcerated | 5 | 2 |
| Ulceroproliferative | 16 | 10 |
| Histopathological Type |
| MD CRC | 23 | 11 | 0.7 |
| PD CRC | 5 | 2 |
| Undiff CRC | 3 | 0 |
| MUC CRC | 3 | 4 |
| SR CRC | 1 | 1 |
| Grade |
| I | - | - | 0.1 |
| II | 25 | 13 |
| III | 5 | 4 |
| IV | 5 | 1 |
| Stage |
| I | 4 | 2 | 0.4 |
| II | 8 | 9 |
| III | 15 | 6 |
| IV | 8 | 1 |
| Neural invasion | 3 | 2 | 0.7 |
| Vascular invasion | 2 | 0 | 0.8 |
| Lymph Node | 18/35 | 7/18 | 0.09 |
| Liver/Bone/Prostate/ peritoneal metastasis | 2/1/1/1 | 2/0/0/1 | 0.8 |
| Survival |
| AWD | 16 | 5 | 0.6 |
| AWoD | 7 | 6 |
| D | 4 | 1 |
| L | 8 | 6 |
AWD- Alive with disease, AWoD- Alive without disease, D- Dead, L- Lost to follow up. P-value >0.05 is considered significant
According to Pearson chi square exact test, neuroendocrine differentiation as a prognostic factor for disease free survival at the end of 3 years was not statistically significant (p>0.5), as majority were alive with or without disease [Table/Fig-4] and Kaplan Meier survival analysis did not show separation between the two groups either.
Discussion
Cancer cells with neuroendocrine differentiation have been observed in gastrointestinal carcinomas. Neuroendocrine differentiation in CRCs is often difficult to diagnose by routine H&E staining. Most commonly used neuroendocrine markers are NSE, Chg A and Syn showing a suitable sensitivity and specificity [4–12]. In present study, Chg A & Syn have been used as a marker for neuroendocrine differentiation on moderately, poorly and undifferentiated CRCs.
The distinction between neuroendocrine carcinoma and adenocarcinoma with neuroendocrine differentiation remains unclear and their definitions have not yet been determined. Studies have suggested that the CRCs with extended expression of neuroendocrine markers should be defined as the neuroendocrine cancer and those containing more than 2% and less than 25% neuroendocrine component as CRC with neuroendocrine differentiation. But usually both nomenclatures are used to define the tumour with expression of neuroendocrine differentiation markers. However, the recent WHO classification [1] recommends use of the term ‘neuroendocrine tumour’ when the tumours contains at least 30% of obviously neuroendocrine cells, while most of the studies [6–9] have taken >2% positivity for neuroendocrine markers as presence of neuroendocrine differentiation. So, in the present study, we further divided the immunoreactivity of Chg A & Syn into 0, <2, 2-10%, 10-30% & >30% and correlated with grade, stage & survival to look for significant differences in the subgroups. The study results did not show any correlation of these subgroups with different parameters.
Furthermore, different studies have classified neuroendocrine differentiation on immunohistochemistry based on three different staining patterns. First of all, diffuse staining of all tumour cells which is mostly seen in poorly differentiated and undifferentiated colorectal cancer. Second, >25% of all tumour cells are positive for neuroendocrine markers which is seen in moderately or poorly differentiated carcinomas. Lastly, >2% tumour cells are positive for neuroendocrine markers. The positive cells scatter in the glandular structure, resembling the neuroendocrine cells in the normal mucosa, however the standard of >2% cut off is taken, as upto 2% neuroendocrine cells can be seen in normal mucosa [4,5,10]. Accordingly, in our present study, neuroendocrine differentiation was seen in 33.9% of cases, with grade 2 positivity in 9.4%, grade 3 positivity in 13.2% & grade 4 positivity in 11.3% of cases.
Neuroendocrine differentiation in tumours of different sites has been an area of ongoing research. Few of the studies [6,7,10–13] have shown that the neuroendocrine differentiation was significantly associated with the grade and stage of the tumour & inversely proportional to the survival. While other studies [14–19] have contradicted these results, suggesting that there was no significant correlation between the neuroendocrine differentiation & survival. Few other studies have shown the higher incidence of distant metastasis either to the lymph nodes [20] or liver [5] or metachronous distant recurrence of CRCs with neuroendocrine differentiation [18]. In our study, 8/53 cases showed distant metastasis in the liver, mesentery, bone & prostate. However, the primary tumour did not show neuroendocrine differentiation in these cases. The significance of neuroendocrine differentiation in various studies has been highlighted in [Table/Fig-5].
Comparison of prognostic significance of NED among different studies
| Author Name and Year | n | Age | Sex | Location | Size | Type | Grade | LN mets | Liver mets | Stage | Survival |
|---|
| Hamada et al., [13] 1992 | 212 | - | - | NS | - | - | NS | - | - | NS | S |
| Mori et al., [16] 1995 | 108 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Syversen et al., [12] 1995 | 91 | - | - | S | - | - | - | - | - | - | S |
| Secco et al., [19] 1997 | 100 | - | - | NS | - | - | NS | - | - | NS | - |
| Lioyd et al., [14] 1998 | 289 | - | - | - | - | - | - | - | - | - | S |
| Grabowski et al., [7] 2001 | 116 | - | - | NS | - | - | NS | - | - | NS | S |
| Grabowski et al., [8] 2002 | 20 | - | - | NS | - | - | NS | - | - | NS | S |
| Indinnimeo et al., [20] 2002 | 56 | - | - | - | - | - | - | S | - | - | - |
| Famulski et al., [17] 2003 | 48 | NS | NS | NS | - | NS | NS | S | - | - | - |
| Atasoy et al., [10] 2003 | 50 | - | - | - | - | - | - | - | - | - | S |
| Grabowski et al., [9] 2004 | 20 | - | - | - | - | - | - | - | - | - | S |
| Mahmoud et al., [11] 2006 | 62 | - | - | - | - | S | S | - | - | S | S |
| Shinji et al., [5] 2006 | 48 | - | - | - | - | - | - | - | S | - | - |
| Schwander et al., [18] 2007 | 94 | - | - | - | - | - | - | - | - | - | NS |
| Shayanfar et al., [6] 2009 | 83 | NS | NS | NS | - | NS | S | - | - | NS | - |
| Cho et al., [15] 2010 | 89 | - | - | - | - | - | - | - | - | - | NS |
| Present study | 53 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
‘NS’ Not Significant, ‘S’ Significant, ‘-’ test not done
Grabowski et al., studied the frequency and prognostic significance of neuroendocrine marker expression in 20 cases of undifferentiated CRCs. Their results showed that neuroendocrine differentiation was often seen in small cell undifferentiated carcinoma and it has a more aggressive course of the disease [8,9]. In the light of the literature and controversy that exists regarding the neuroendocrine differentiation in CRCs, this study was planned to analyse the prognostic significance of neuroendocrine expression in CRCs.
IHC (Chg A & Syn) was performed in 53 cases of CRC. Out of which, 27% and 8% of cases showed positive reaction (>2% of tumour cells) for Chg A & Syn respectively, suggesting that Chg A was a more sensitive marker than Syn. Syversen et al., reported Chg A and NSE immunostaining in 15% and 36% of tumours, respectively [12]. Furthermore, they found that the expression of NSE was significantly higher in CRC derived from the mid gut than in those of hind gut origin. Similarly, Shayanfar et al., found Chg A and NSE immunostaining in 38 and 26% of tumours, respectively (n=50cases) [5]. Atasoy et al., observed Chg A expression in 38%, NSE expression in 26%, and Syn expression in 6% of the tumours [10].
Adenocarcinoma was the most common histological type in most of the studies [6,11] which was in consensus with our study along with the stage at presentation being stage II or III. One of the highlights of the study was that we found neuroendocrine marker positivity in 4 out of 7 (57%) mucinous carcinomas, suggesting a higher incidence of neuroendocrine positivity in mucinous tumours which was comparable with the study by Shayanfar et al., [6].
Some studies revealed that the poor prognosis of CRC with neuroendocrine differentiation is related with marked tumour invasion of lymphatic and veins resulting in liver metastases, aggressive nature, lymph node and distant metastasis [4,11,20]. In the present study, we found 4 cases (8%) with hepatic metastasis, 2 cases (4%) of peritoneal metastasis and one each of bone & prostate metastasis. However, as compared to the non-neuroendocrine differentiation group, these features did not show any significant difference.
A number of molecules such as vascular endothelial growth factor, cycloxygenase etc. have been studied in colorectal carcinomas and hence the specific target therapy have been discovered. These drugs are presently being used and are having an impact on improving the morbidity & mortality rates in CRCs. Similarly neuroendocrine differentiation is being studied for relevant targeted therapy. As such there has been no standard therapy for CRC with neuroendocrine differentiation. Some of the suggested treatment strategies are hormone analogues secreted by tumour cells with neuroendocrine differentiation, biotherapy with interferon alpha, somatostatin analogues & chemotherapy [4]. However, because the study was retrospective, all the colorectal carcinomas were given the same chemotherapy.
The molecular basis of neuroendocrine tumourigenesis is an area of ongoing research. Increased knowledge of the molecular pathogenesis for the development of NE differentiation may improve the management of these tumours in the future. Gastrointestinal neuroendocrine tumours & tumours with NE differentiation are known to be associated with familial & genetic syndromes such as neurofibromatosis, multiple endocrine neoplasia syndrome 1 (MEN1) and von Hippel-Lindau disease. Some studies have shown the absence of both classic oncogenes (scr, ras, myc, fos, jun) and suppressor genes (P53, RB) while some other studies showed CpG island methylator phenotype (CIMP) positivity in CRCs with NE differentiation. Latest technologies should be used to explore the newer molecular markers for specific diagnosis & development of targeted therapy for these NE tumours & tumours with NE differentiation [4,21].
Conclusion
It is quite controversial whether CRCs that richly express neuroendocrine markers have the same prognosis as those devoid of this expression. With a limited number of studies addressing this question, our study proposes that neuroendocrine differentiation in CRCs does not have significant association with age, sex, histological type, grade, stage, survival, lymph node metastasis or distant metastasis. Hence, to conclude, neuroendocrine differentiation in colorectal carcinomas does not have a prognostic significance. A convincing relationship can only be settled when a large number of colorectal cancers from different cohorts could be put into a retrospective study. Further study of the area should be addressed to give a clear clue to evaluate the significance of neuroendocrine differentiation in CRC, with effect to the targeted therapy and improved survival.
List of Abbreviation
CRC: Colorectal carcinoma,
Chg A: Chromogranin A,
Syn: Synaptophysin,
NSE: Neuron Specific Enolase,
IHC: Immunohistochemistry,
AWD- Alive with disease, AWoD- Alive without disease, D- Dead, L- Lost to follow up. P-value >0.05 is considered significant
‘NS’ Not Significant, ‘S’ Significant, ‘-’ test not done