Optimal treatment strategy in children with Hodgkin Lymphoma (HL) remains controversial, especially in cases of advanced disease. Treatment modalities have varied from total nodal radiation therapy (RT) to Combined Chemo–Radiotherapy (CMT) and to Chemotherapy (CT) alone [1–4]. For the last two decades, CMT regimens using less toxic CT and low dose local RT has been the treatment modality preferred by most paediatric study groups. The aim is to minimize late complications of treatment while maintaining high cure rates [5–9], with 5-year Overall Survival (OS) rates >95%, and 5-year EFS (event free survival) > 90%. More recently, there is a tendency to use CT alone, particularly in younger children, so as to avoid long-term sequelae due to RT, especially premature epiphyseal fusion and secondary malignancies [10,11]. We analysed the OS as well as EFS of all stages of childhood HL treated with chemotherapy as per our unit protocol, using cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) or alternating cycles of COPP (cyclophosphamide, vincristine, procarbazine, and prednisone) and ABVD.
Materials and Methods
All children <13 years age diagnosed with HL on biopsy presenting to our centre during the study period were eligible for inclusion. Those with concurrent HIV infection, any pre-existing severe organ (kidney, liver, cardiac, cerebral) dysfunction were excluded.
Disease Staging
The pretreatment evaluation included a detailed patient history and physical examination. Routine laboratory studies including Complete Blood Cell Count (CBC) with differentials (DLC), Erythrocyte Sedimentation Rate (ESR), renal and liver function tests (RFTs and LFTs), serum uric acid, and lactate dehydrogenase (LDH) level were obtained for all children before treatment. All patients had undergone chest x-ray (CXR), bone marrow aspiration/biopsy (except in stage IA - IIA), contrast enhanced Computerized Tomography Scan (CECT) of chest, abdomen and pelvis. PET scan was done whenever there was a discordance between CT findings and clinical and laboratory presentation, or a concern of CT overestimating disease extent due to inflammation and fibrosis. Lymphangiogram, gallium scan and staging laparotomy were not done in any of our subjects. Splenic involvement was defined as clinically detectable splenomegaly or presence of single or multiple hypodense lesions on abdominal CECT or presence of single or multiple hypoechoic splenic lesions on ultrasound examination of the abdomen. Clinical staging was determined according to the Ann-Arbor Classification [12]. Bulky disease was defined as presence of lymph nodal mass of at least 6 cm diameter or a mediastinal mass with a diameter exceeding one third of the maximum intrathoracic cavity width on an upright postero-anterior chest radiograph. Patients were histologically classified according to the Ryes modification of Lukes and Butler scheme [13].
Treatment Protocol
After staging workup, all patients with early stage disease (I-II) were stratified into favourable and unfavourable groups, based on B-symptoms and bulky disease. Early stage favourable patients (IA, IIA) received 2-4 cycles of ABVD (with or without involved field RT of 20-30Gy), and early stage unfavourable patients (IB, IIB) were treated as advanced disease and received 4 cycles of ABVD (with or without involved field RT). All patients with late stage disease (stage III and higher) received 6 cycles of ABVD (with or without involved field RT). Severity of disease was defined to include those of early stage unfavourable disease (stage IB, IIB) and late stage disease (stage (III to IV).
Clinical Re-Staging
Patients were reassessed for response after 2-4 cycles of ABVD, by a detailed physical examination, blood investigations, tissue examination from any new lymph nodes and repeat CT scan of the original site of involvement. PET scan was done if there was discordance between CT findings and clinical presentation, or a concern of CT overestimating disease extent due to inflammation and fibrosis.
Complete Remission (CR) was defined as the complete regression of clinical and radiological lesions. Persistence of some radiological abnormalities at the site of previous disease, such as minimal mediastinal widening, was considered as unconfirmed CR until close surveillance showed absence of increase of the radiological abnormalities. Partial Remission (PR) was defined as the reduction in all disease sites by at least 50% compared with the initial involvement. Disease progression was defined as increase by ≥25% of at least one measurable lesion, or by the appearance of a new lesion. In case of complete remission, two more cycles of chemotherapy were given. PR was treated with two cycles of chemotherapy, followed by re-evaluation. Localized residual disease was treated with involved field RT, and widespread residual disease was treated with high dose CT (BEACOPP). In case of non responsive or progressive disease, histopathology was reviewed, and high dose CT followed by stem cell transplant was considered.
Assessment of Outcome and Toxicity
When a patient was lost to follow-up, the time of most recent follow-up examination was used. Variables assessed were – complete response (CR) after four cycles, Partial Response (PR) or relapse (REL), development of secondary malignancy, and death. Measurements of growth and development, assessment of cardiac, pulmonary, and thyroid functions were performed as per the unit protocol. Baseline evaluation of cardiac function was performed by Electrocardiogram (ECG), echocardiography (ECHO), and Multi-Gated-Acquisition (MUGA) Scan. Ejection Fraction (EF) <50% was considered abnormal. Pulmonary Function Tests (PFTs) like total lung capacity, vital capacity, residual volume, and measurement of the diffusing capacity of the lung for carbon monoxide (DLCO) were measured. Thyroid function test was performed only if RT was administered, and included measurement of thyroid stimulating Hormone (TSH) and free thyroxin (FT4). Thyroid dysfunction was defined by an elevated TSH level (above the upper limit of normal).
Follow-Up
After completion of therapy, all patients were closely followed-up for disease relapse, or side-effects of CT/RT. Thorough clinical history and examination (including measurement of weight and height) was performed at each visit (2-4 monthly during the first 1-2 years, and then 3-6 monthly for 3-5 years). CXR or CECT was performed every 6-12 months for 2-5 years after completion of treatment. CT scan of abdomen and pelvis was done, when indicated. TSH levels were checked annually in those receiving RT. Children who had received blood transfusions during chemotherapy were evaluated for HIV and HBsAg status once they were in CR. Echocardiography and pulmonary function tests were done for all the patients.
Statistical Analysis
Event free survival (EFS) was calculated from the initiation of treatment to relapse, progression of the disease, or the most recent follow-up examination. Overall survival (OS) was determined as the time from initiation of therapy to death or the last follow-up examination. Patients who died as a result of causes other than HL without evidence of recurrence at the time of death were censored. Besides descriptive statistics, the univariate analysis for qualitative data was carried out by applying Chi-square (χ2) or Fisher exact test, wherever applicable, for comparing two groups. Besides this relative risk (RR) along with 95% confidence interval (CI) was also calculated. Multivariate logistic regression model and the Cox regression model were performed to determine independent prognostic variables. When follow-up was also considered, the survival analysis was carried out. Survival curves were generated with the Kaplan-Meier actuarial survival method [14]. The comparison between two survival curves was carried out by using log rank test. The signification was observed at P-value <0.05. All calculations were performed with SPSS version 15.0 statistical software (SPSS Inc, Chicago, IL).
Results
Patients’ Characteristics
A total of 35 patients were included in the analysis and their characteristics are depicted in [Table/Fig-1]. There were 31 boys and four girls (M:F ratio 7.6: 1). The median age was 8.3 years (range, 3.5 to 13). The main symptom at presentation was painless progressive enlargement of cervical lymph nodes (96%). The median duration of symptom was nine months (range, 1 to 20). The laboratory parameters are given in [Table/Fig-2]. CECT of the abdomen was performed in all cases. PET CT scan was done in 10 patients. 24 children (68.6%) were <10-year-old, and 23 (65.7%) presented with late stage disease (stage III, and IV). A total of 18 children (51.4%) had bulky disease at presentation, of whom 9 (25.7%) had bulky mediastinal disease. Viral markers (HBsAg, HCV and HIV) were negative in all the patients. Bilateral cryptorchidism and incidental ileo-ileal intussusceptions was detected in one child each. One child underwent splenectomy in view of non-confirmatory lymph node biopsy results, and evidence of splenic involvement with HL.
Characteristics of study population
| Characteristics | Number (N) | Percentage (%) |
|---|
| Sex Male Female | 3104 | 88.611.4 |
| Age ≤ 5 years > 5 – 10 years >10 – 13 years | 022211 | 5.762.931.4 |
| Clinical findings Peripheral lymphadenopathy Bulky disease Mediastinaum (M) Peripheral (P) Both of the above (P+M) B symptoms Spleen involvement Extranodal disease (other than spleen involvement) | 35091921212106 | 10025.754.360606017.1 |
| Histologic subtypes Mixed cellularity (MC) Nodular sclerosis (NS) Lymphocyte predominant (LP) Unclassified (UC) | 09100907 | 25.728.625.720 |
| Clinical stageIIIIIIIV | 03091805 | 8.525.851.414.2 |
| Characteristics | Number (N) | Percentage (%) |
|---|
| Hemglobin (gm/dl) <11 ≥11 | 2510 | 71.428.6 |
| WBC count (per cu mm) <5000 5000-12,000 >12,000 | 052604 | 14.374.311.4 |
| ESR (mm/hour) <40mm ≥40mm | 1916 | 54.345.7 |
| LDH level (IU/L) <350 ≥350 | 1025 | 28.671.4 |
| A: G ratio reversal | 08 | 22.8 |
| Transamnitis | 01 | 2.8 |
WBC - white blood cell count; ESR - erythrocyte sedimentation rate; LDH - lactate dehydrogenase; A: G ratio - albumin: globulin ratio
Treatment Response
ABVD regimen was started initially in every patient irrespective of the staging status. Additional cycles of COPP were given depending upon whether there was partial remission, relapse, non-response or progression of the disease. Two children (one each in stage III and stage IV) received BEACOPP regimen due to progression of the disease on initial regimens. Four patients (11.4%) were given additional RT for residual disease. All patients were evaluated for treatment response as shown in [Table/Fig-3]. After 4-cycles of CT, 30 patients (85.3%) showed CR, while 5 patients (14.7%) showed partial remission. Four patients (11.4%) showing CR at the stage of reassessment relapsed later at follow-up durations of 11, 20, 31 and 52 months, respectively. One child died during therapy unrelated to the disease process or treatment (due to massive pulmonary haemorrhage secondary to pulmonary tuberculosis). The characteristics of the 5 children who attained partial remission are shown in [Table/Fig-4a]. The details of two patients who relapsed are shown in [Table/Fig-4b].
Response to chemotherapy in relation to sex, age, histology and stage of disease
| Variables | Initial therapy | CR after subsequent therapy |
|---|
| CR | PR | REL | CT | CT+RT |
|---|
| Sex Male Female | 2404 | 05- | 02- | 02- | 05- |
| Age ≤ 5 years >5-10 years >10-13 years | 011809 | 010301 | -0101 | -02- | 010301 |
| Stage (Ann Arbor) I II III IV | 011809 | 010301 | -0101 | -02- | 010301 |
| Histology (Rye) MC NS LP UC | 08070904 | 0101003 | -02-- | -01-01 | 0102-02 |
The clinical and therapeutic characteristics of 5 children in partial remission
| Age (yrs) | Sex | Histology | Stage | “B” symptom | Therapy | Status |
|---|
| 8 | M | NS | 1 | absent | CT+RT | Alive |
| 8.2 | M | MC | 3 | present | CT+RT | Alive |
| 3.5 | M | UC | 1 | absent | CT+RT | Alive |
| 12 | M | UC | 2 | present | CT+RT | Alive |
| 10 | M | UC | 3 | present | CT+RT | Alive |
The clinical and therapeutic characteristics of 2 children who relapsed
| Age (yrs) | Sex | Histology | Stage | “B” symptom | Duration 1st CR (months) | Relapse | Duration 2nd CR (months) | Relapse |
|---|
| 6 | M | NS | 3 | present | 20 | CT+RT | 6 | Alive |
| 13 | M | NS | 3 | present | 26 | CT (high dose) | 45 | Alive |
Survival
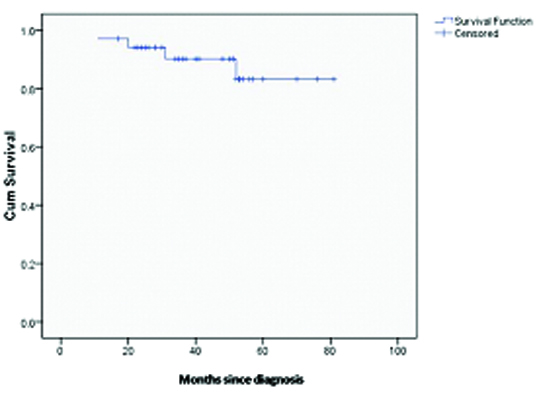
OS and EFS were estimated for all the patients. One patient in CR died due to an unrelated disease (pulmonary tuberculosis). The mean (SD) duration of follow-up was 46.3 (16.3) months. Thirty-two patients that are alive are in CR (includes 2 patients who are in second remission after treatment for relapse). Five patients (14.7%) who demonstrated partial remission on CT (on initial assessment) were followed up with additional doses of CT and low-dose involved field RT (20–30 Gy) [Table/Fig-4a&4b]. These five patients had following features: spleen involvement (n=2), bulky mediastinal disease (n=3), bulky peripheral disease (n=2), and bulky peripheral plus mediastinal disease (n=3). None of these patients have died due to disease and there were no patients that were lost to follow up (the projected OS at 5 years being 100%). The mean (SE) EFS was 73.1 (3.7) months. The median could not be calculated, because of less number of events as well as early occurrence of events. With a median follow-up duration of 26.5 months, the projected EFS at 5 years is 76.7% (SE 3.6%) [Table/Fig-5]. The EFS according to clinical stage of the disease is given in [Table/Fig-6]. Various prognostic parameters were studied for survival [Table/Fig-7]. Univariate analysis showed significantly lower EFS in those having advanced stage disease (p = 0.037), bulky mediastinal disease (p = 0.015), spleen involvement (p = 0.049), and high LDH level (p = 0.048). On multivariate analysis, none of these factors showed any prognostic significance (p-value of each of the variables ≥ 0.995), which may be due to small sample size or significant interaction between the variables [spleen involvement and LDH level (p = 0.009), spleen involvement and bulky mediastinal disease (p = 0.001), advanced stage and LDH level (p = 0.009), advanced stage and bulky mediastinal disease (p = 0.009), LDH level and bulky mediastinal disease (p = 0.009)].
Overall Event-free survival (EFS) in all children
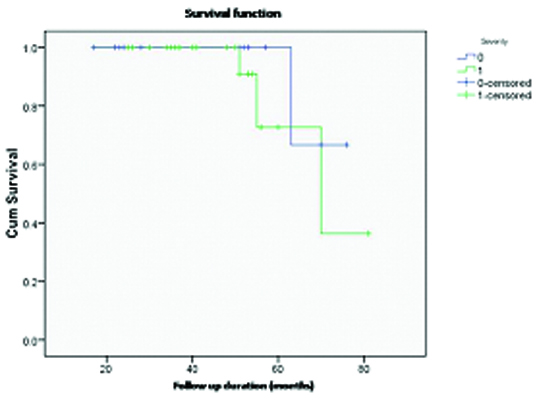
Event-free survival according to severity of disease
Univariate Analysis of Various Prognostic Factors for Event-Free Survival (EFS) by Cox Regression
| Variables | Number of patients | Number of events | RR | 95% CI | p-value |
|---|
| Sex Male Female | 3104 | 70 | - | - | 0.548 |
| Age < 10 years ≥10 years | 2411 | 43 | 0.4711 | 0.081 – 2.743 | 0.64 |
| Clinical stage I II III IV | 06101801 | 2140 | N/A | N/A | 0.741 |
| Early or late stage Early (I-II) Late (III-IV) | 1223 | 43 | 6.331 | 1.001 – 40.071 | 0.037* |
| B symptoms Present Absent | 2114 | 43 | 1.0671 | 0.149 – 7.145 | 0.947 |
| Lymph node involved ≤2 >2 | 1124 | 16 | 0.1821 | 0.019 – 1.759 | 0.193 |
| Bulky mediastinal disease Yes No | 0926 | 52 | 15.0251 | 1.677 – 134.572 | 0.015* |
| Bulky disease Yes No | 2114 | 52 | 8.2131 | 1.008 – 66.914 | 0.049* |
| Histologic subtypes MC NS LP UC | 09100907 | 1312 | N/A | N/A | 0.165 |
| Haemoglobin (g/dl) <11 ≥11 | 2510 | 43 | 0.371 | 0.062 – 2.23 | 0.269 |
| WBC count (per cu mm) <12,000 ≥12,000 | 2609 | 61 | 0.5711 | 0.044 – 7.438 | 0.666 |
| ESR (mm/hour) <40mm ≥40mm | 1916 | 16 | 0.3811 | 0.038 – 3.784 | 0.398 |
| LDH level (IU/L) <350 ≥350 | 1220 | 43 | 8.6951 | 1.015 – 74.503 | 0.048* |
| Hypoalbuminemia Present Absent | 0827 | 34 | 0.3811 | 0.122 – 1.193 | 0.596 |
| Spleen involvement Present Absent | 2114 | 52 | 8.2131 | 1.008 – 66.914 | 0.049* |
| Extranodal disease (other than spleen involvement) Present Absent | 0629 | 16 | 0.3811 | 0.038 – 3.784 | 0.638 |
| Chemotherapy ABVD ABVD-COPP | 1619 | 25 | 0.6221 | 0.099 -.3.923 | 0.612 |
*p-value <0.05. N/A, not applicable
Toxicity and Complications
These have been compared in [Table/Fig-8]. Nearly all patients developed complete but temporary alopecia. Myelosuppression was mostly mild to moderate. Only 14% patient received haematopoietic growth factors, but 40% required one or more blood transfusions. The commonest complications were infections, febrile neutropenia, (14%), herpes zoster (2.8%), and tuberculosis (2.8%). Amongst the side-effects of CT regimen used, neither symptomatic cardio-toxicity (all had normal EF at the end of chemotherapy, minor tricuspid and mitral regurgitation was noted in 2 patients) nor symptomatic pulmonary disease (2 patients developing reversible mild asymptomatic restrictive pattern in the PFT attributable to bleomycin) was noted in any of the patients. Of the 5 patients who received RT, only 1 patient developed hypothyroidism. Majority of the patients received therapy in an outpatient setting, so that they were able to attend the schools. No sex dependent toxicities were noted.
Comparison of toxicity of chemotherapy and radiotherapy
| Toxicity | Chemotherapy group (n=35) | Radiotherapy group (n=05) |
|---|
| Alopecia | Common | None |
| Myelosuppression Anaemia (requiring transfusion) Requirement of haematopoietic growth factors Febrile neutropenia | 40%14%14% | NoneNoneNone |
| Tuberculosis | 2.8% | None |
| Herpes zoster | 2.8% | None |
| Cardiotoxicity (mild) | 2.8% | None |
| Pulmonary toxicity (reversible) | 2.8% | None |
| Hypothyroidism | None | 20% (1 out of 5) |
Discussion
Over the last three decades significant changes have occurred in the management of paediatric HL. Success of combination CT in late stages of HL and delayed side effects of RT in children directed us to initiate chemotherapy in all the stages of the disease. Moreover, staging laparotomy with its complications and morbidity could be avoided. The results of our study showed a strikingly high male to female (M:F) ratio (7.6:1) which is in accordance with previous Indian studies [10,15–17]. The mean age at diagnosis was 8.3 years, which is similar to that found in previous study in children with HL [3]. Nearly 70% of our patients were ≤10-year-old.
There are few reports on histological subtypes of HL in Indian children. In a previous Indian study, 83% had mixed-cellularity subtype, which did not have any significant adverse impact on the survival. In contrast, children in our study have nodular-sclerosis as the most common histological subtype, though no adverse impact on survival was noted. The absence of prognostic significance of histology in childhood HL was shown in a recent study conducted by the UK Children’s Cancer Study Group [18]. Advanced stages were seen in nearly 66% of our cases, which is similar to previous reports from India [3,10,15,19]. This could be partly explained by the misled presumptive diagnosis and treatment of lymph node tuberculosis, and delayed referrals in case no response to anti-tubercular drugs.
The identification of prognostic factors facilitates the definition of risk groups for risk-adapted therapy. However, few studies have analysed prognostic factors for paediatric HL [3,10,20–24]. In the study by Smith et al; male gender, advanced stage, bulky mediastinal disease, haemoglobin (Hb) <11.0 g/dl and WBC count > 11.5 × 103/cumm were the factors that independently predicted inferior EFS and OS rates [20]. In the study by Koga et al., High-risk disease and age (>10 years) were considered to be poor prognostic factors [21]. In another study, Vecchi et al., reported nodular-sclerosis histology, B symptoms, and large mediastinal mass as unfavourable prognostic factors [22]. Two paediatric studies have analysed various prognostic factors in advanced HL. The Paediatric Oncology Group (POG) found stage IV disease and male sex to be associated with inferior EFS [23], whereas the Children’s Cancer Group found high ESR, hepatomegaly, and mediastinal bulky disease among stage III patients, as negative prognostic factors [24]. In our study, advanced stage, bulky mediastinal disease, bulky disease, involvement of spleen and high LDH level (≥ 350 IU/L) were all prognostic factors for inferior EFS on univariate analysis. LDH level > 320 Iu/ml as an adverse prognostic factor for both EFS as well as OS has been found in one study [25]. Small patient population and no deaths precluded the evaluation of some of the prognostic factors the present study. The lack of significance of some of the prognostic factors may be due to various study factors such as small sample size, very good response to chemotherapy [2,19,26,27] and/or use of FDG-PET/CT scan for better disease delineation [28]. The haemoglobin (Hb) level for prognostic relevance may need revision in view of the high prevalence of anaemia in the Indian subcontinent.
Our results are comparable to previous studies using chemotherapy alone [2,3,10,19,29,30]. Only two prospective trials [25,26] have compared CMT versus CT in paediatric HL, but have not shown any statistical superiority of CMT over CT (though survival was higher in CMT) [25]. A meta-analysis comparing CT versus CMT suggested a limited role for RT [31], while a recent study emphasized CMT to be the standard of care in paediatric HL [32]. This latter conclusion is based on the improved EFS of those treated with RT compared with those who get CT only. However at a follow-up of 3 years, there was no difference in OS between both the groups. Second malignancies (of the breast and thyroid) due to RT also occurred > 3 years after therapy in many patients [33]. A recently completed long term follow up study (including patients > 16 years age) found that, at a median follow-up time of 11.3 years, the rate of OS was lower with subtotal nodal radiation therapy, with or without two cycles of ABVD, than with ABVD alone (hazard ratio for death with ABVD alone, 0.50; p = 0.04) [34]. This difference was due to the number of deaths from causes other than HL, including second malignancies. The total number of second malignancies (breast, thyroid, gastrointestinal, leukemia, soft-tissue sarcoma, bone, CNS, Lymphoma, melanoma, head & neck, lung, and skin) and cardiovascular events was higher in the RT group than in the ABVD-alone group. Our data in combination with those of the others mentioned above do not support CMT as the gold standard for initial therapy in childhood HL.
The CT regimens have both acute (myelosuppression) and long-term (cardiac, pulmonary, gonadal, and secondary leukemia) organ toxicities. Regimens alternating ABVD with COPP regimens improve disease control and reduce the cumulative dose of alkylating agents, but increase the risk of long-term cardiopulmonary toxicity [35–37]. In our study (mean follow-up of 46.3months) as well as in previous studies [25,26], the follow-up period is still too short to fully appreciate the late adverse-effects. Because most recurrences after CMT for paediatric HL tend to occur during initial 3 years of treatment [5–9,23,38,39], long-term outcomes would not change considerably by increasing the length of follow-up. Worth mentioning is the difference in the EFS and the OS recorded by our unit, which suggests that, we were able to salvage many patients despite the presentation at more advanced stages.
Only four children required additional RT for remission with the OS being 100%. The results show that RT could be used as a second line effective salvage therapy if CT is not sufficient. Thus additional RT could be reserved for those cases that present with residual disease or relapse after completing chemotherapy, which also has been shown by other studies [19].
At the moment we have been able to evaluate cardiac, thyroid, and pulmonary function in all children. No clinical signs of cardiac dysfunction have been found in these patients. This may be due to considerably lower dose of doxorubicin used in our regime. It is worthy to note that this lower dose has not adversely affected clinical outcomes, while avoiding serious side-effects.
There is a role of Autologous Stem Cell Transplant (ASCT) in children with relapsed/refractory lymphoma HL. In a recent study, approximately half of all paediatric patients with lymphoma who failed first-line therapy and demonstrated chemosensitivity to second-line therapy could be salvaged with ASCT. Though, it was planned in present study, none of our patients underwent ASCT due to multiple reasons [40].
Limitations
There are some limitations of our study. Small sample size precluded derivation of prognostic factors. A short follow up period was also a disadvantage, though none of our patients were lost to follow-up. Despite these limitations, our study has shown that CT alone (with ABVD or alternating COPP-ABVD) is as effective as CMT. A longer follow-up is required to see late effects of CMT, including survival, and the quality of life of survivors.
Conclusion
Present study found systemic CT alone to be an effective therapy in childhood Hodgkin lymphoma. However, a small sample in present study limits the generalisability of these findings. The findings needs to be replicated in larger population, preferably randomized clinical trials, before any firm conclusion can be made.