Premature CAD is defined as cardiac events occurring in men before the age of 45 years and women before the age of 55 years [1]. Coronary artery disease (CAD) is major health problem all over the world. CAD has high mortality, morbidity and is the most common cause of death worldwide. Prevalence rate of CAD in India is manifold higher than that in the developed countries and prevalence is gradually rising in the last few decades [2]. The prevalence of CAD within the Indian Subcontinent has increased by a factor of 10 within the last 50 years leading to CAD epidemic in India. [3] According to the National Commission of Macroeconomics and Health there will be 62 million CAD patients in India and out of this 23 millions CAD patients will be below 40 years of age by the end of 2015 [4]. The prevalence of CAD is not only very high in Indian subcontinent but also in migrant Asian Indians. Migrant Indians in USA have 4 times prevalence rate than native white Americans [5–7]. Coronary artery disease occurs prematurely and is more severe in the Indian population as compared to any other ethnic group in the world [5–7]. The number of deaths has doubled in India since 1985. Moreover deaths related to CAD occur 5-10 years earlier in Indian subcontinent [8]. As one sixth of world’s population reside in India and there is high fraction of younger age groups currently in India, the high occurrence of CAD puts great burden on the Indian population.
High rate of CAD in Indians is attributed to genetic predisposition and environment [9–11]. Indian population is genetically susceptible to CAD from early childhood which can only be partially explained on the basis of higher level of atherogenic lipoprotein (a) and lower level of anti-atherogenic HDL-C [12] in Indians population as compared to any other ethnic groups. Lipoprotein (a) is not only better marker of CAD but also independent risk factor for premature CAD. The CAD risk is increased many times by the presence of other risk factors like diabetes, hypertension, smoking, sedentary lifestyle, abnormal lipids, i.e. higher level of total cholesterol, TG, LDL-C and lower level of HDL-C. Increased LDL-C and decreased HDL-C are known risk factors for CAD [13]. But the high rate of prematurity, morbidity and mortality can not be explained solely on the basis of conventional lipid parameters as many studies have shown normal levels of LDL and HDL in CAD patients. The normal level of LDL in such patients cannot explain the occurrence of CAD. Therefore lipid risk factors other than high LDL and low HDL might be deeply involved in the development of CAD, and which can explain occurrence of premature CAD in India. Therefore we have studied advanced lipid parameters i.e. oxLDL. Sd LDL. Lp (a), ApoA1 and ApoB in our study along with conventional lipid parameters. Small dense fraction of LDL and oxidised LDL can not only explain the risk of CAD in normolipidemic patients but also correlated with mortality and morbidity of CAD which is dependent upon absolute LDL number of foam cells which in turn dependent upon level of ox LDL. Therefore, levels of small dense LDL and ox LDL will help in early detection and intervention in CAD patients. They will be focus of targeted drug therapy as decrease in levels of small dense LDL and ox LDL rather than LDL-C will decrease the risk formation of foam cells and occurrence of acute coronary events.
Materials and Methods
After obtaining necessary clearance from the Institutional ethical committee, we conducted this study in a tertiary care multispecialty hospital attached to a Medical College in New Delhi from January 2009 to April 2009.
Inclusion Criteria
Thirty premature coronary artery disease patients suffering from stable angina, unstable angina or acute myocardial infarction (males of the age less than 45 years and females of the age less than 55 years) admitted to the medical emergency and coronary care unit (CCU) of tertiary care Hospital in New Delhi were taken as cases for our study. The premature CAD patients were diagnosed on the basis of detailed history, clinical examination, ECG changes, cardiac injury markers (Troponin T, CK-MB fraction) and echocardiography.
Thirty age and sex matched healthy persons were taken as controls. Informed and written consent was taken from all the subjects in case and control groups before the study.
Exclusion Criteria
Cases and controls were excluded if they had any concurrent infection (acute or chronic), chronic liver disorders, chronic kidney disorder or history of intake of hypolipidemic drugs, oral contraceptives or hormone replacement therapy.
Collection of Blood Samples
Venous blood samples of all the subjects under study were collected after an overnight fasting taking all aseptic precautions. The blood was subjected to centrifugation at 3500 rpm for ten minutes and the separated serum was used for the estimation of routine lipid profile, ox LDL, Lp (a), ApoA-1, Sd LDL and ApoB (ApoB-100). In the fasting state, most of the ApoB is in ApoB-100 state
Total cholesterol & Triglycerides were estimated by using commercial available kits (Accurex Biomedical Pvt Ltd, Mumbai, India). LDL-C was calculated by using Friedwald’s equation. Ox LDL was estimated using commercially available ELISA kit (Mercodia, Sweden) which is based on the direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the oxidized apolipoprotein B molecule. Lp (a) was estimated using commercially available ELISA kit (IMMUNOZYM Lp (a) from Progen Biotechnik GMBH, Germany. Apo A-1 and ApoB-100 was measured by using Immunoassay based immunoturbidimetric kits (Diasys). The immunoturbidimetric method was performed on the autoanalyser ‘Olympus AU 400’. Small dense LDL is calculated indirectly by the ratio of the LDL-C/LDL-Apo B. in this formula LDL is calculated by the formula given by Hattori et al., [14].
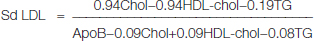
Then their ratio is taken as the indicator of small dense LDL. Lower ratio indicates the preponderance of small dense LDL. Ratio <1.20 indicates high concentration of Sd LDL.
Statistical Analysis
Statistical analysis of the data was carried out using computer based statistical software SPSS 10.0 software (SPSS Inc., Chicago, IL, USA). Values were reported as mean and standard deviation. Correlation between different parameters with premature CAD and with each other was obtained by Pearson’s correlation coefficient, which lies between -1 to +1. A two-tailed p-value <0.05 was considered statistically significant.
Results
The age of the cases varied from 26-54 years with a mean age of 42.47 years. The age of the controls varied from 27-52 years with a mean age of 41.13 years [Table/Fig-1]. The premature CAD was more prevalent in male population with male to female ratio of 2:1 [Table/Fig-2]. Out of the 7 female cases 6 were in the age group of 46-55 indicating lower incidence of CAD in pre-menopausal females.
Age groups in cases and controls
| Groups |
|---|
| Control | Case |
|---|
| No. | % | No. | % |
|---|
| AgeGroups(Years) | 26-35 | 3 | 10 | 1 | 3.33 |
| 36-45 | 22 | 73.33 | 23 | 76.67 |
| 46-55 | 5 | 16.67 | 6 | 20.00 |
Sex distribution in cases and controls
| Sex |
|---|
| Female | Male |
|---|
| Group | Case | 10 | 20 |
| 33.3% | 66.7% |
| Control | 12 | 18 |
| 40.0% | 60.0% |
TC, TG and LDL-C were higher and HDL was lower in cases then controls [Table/Fig-3]. The values of TC, TG, LDL-C and HDL-C in controls were 163.43±17.55 mg/dl, 163.57±20.36 mg/dl, 88.05±13.23 mg/dl and 42.67±4.73 mg/dl respectively. The values of TC, TG, LDL-C and HDL-C in cases were 193.53±40.53mg/dl, 183.10±43.2 mg/dl, 115.67±36.56 mg/dl and 38.10±5.10 mg/dl respectively.
Comparison of conventional and advanced lipid parameters in cases and controls.
| Parameter | ControlsMean±SD | CasesMean±SD | p-value | Statisticalsignificance |
|---|
| Total Cholesterol(mg/dl) | 163.43±17.55 | 193.53 ±40.53 | .001 | Significant |
| TG (mg/dl) | 163.57±20.36 | 183.10 ± 43.2 | .032 | Significant |
| LDL-C (mg/dl) | 88.05±13.23 | 115.67 ± 36.56 | .0001 | Significant |
| HDL-C (mg/dl) | 42.67±4.73 | 38.10 ± 5.10 | .001 | Significant |
| oxLDL (U/L) | 32.43±6.73 | 62.27±8.75 | <0.0001 | Significant |
| Lp(a) (mg/dl) | 17.62±3.18 | 43.17 ± 10.23 | <0.0001 | Significant |
| Apo A-1 (mg/dl) | 111.84±13.40 | 92.73 ± 15.14 | <0.0001 | Significant |
| ApoB (mg/dl) | 73.13±12.86 | 123.07 ± 20.74 | <0.0001 | Significant |
| sd LDL (no units) | 1.46±0.25 | 1.20±0.35 | <0.0001 | Significant |
| ApoB/Apo A-1 | 0.74±0.14 | 1.37±0.34 | <0.0001 | Significant |
Where oxLDL= oxidized low density lipoprotein, Lp(a)= lipoprotein(a), Apo A-1 apolipoprotein A-1
ApoB apolipoprotein B-100, sd LDL= small dense low density lipoprotein
TG= triglyrides, HDL-C=high density lipoprotein-cholesterol, LDL-C= low density lipoprotein-cholesterol
Difference between the two groups was highly significant for TC (p-value <0.001), LDL-C (p-value <0.0001), HDL-C (p-value <0.001) and TG (p-value <0.032). Hence in our study, TG has shown least significance among conventional lipid parameters.
In our study the values of oxLDL, Lp (a), Apo A-1, ApoB and SdLDL in controls were 32.43±6.73 U/L, 17.62±3.18 mg/dl, 111.84±13.40 mg/dl, 73.13±12.86 mg/dl and 1.46±0.25 (no units) respectively. The values of oxLDL, Lp (a), Apo A-1, ApoB and SdLDL in cases was 62.27±8.75U/L, 43.17 ± 10.23 mg/dl, 92.73 ± 15.14 mg/dl, 123.07 ± 20.74mg/dl and 1.20±0.35 (no units) respectively.
So in our study oxLDL, Lp (a), Apo A-1, ApoB and SdLDL are significant statistically (p-value <.0001). ApoB/Apo A-1 ratio in controls and cases was 0.74±0.1 and 1.37±0.3 respectively and was statistically significant (p-value <0.0001).
We also found correlation coefficient of advanced lipid parameters with premature CAD [Table/Fig-4].
Correlation coefficient of advanced lipid parameters with premature CAD
| Correlation coefficient |
|---|
| ox LDL | +0.89 |
| Lp(a) | +0.86 |
| Apo AI | -0.56 |
| Apo B | +0.79 |
| Sd LDL | +0.41 |
oxLDL have shown highest correlation coefficient (r=+0.89) followed by Lp (a) (r=+0.86) and ApoB (r=+0.79).
[Table/Fig-5] shows that ox-LDL were raised in the patients who have normal LDL levels showing that oxidation of LDL is more important as compared to absolute LDL levels
Comparison between LDL values with oxLDL
| Ox-LDL (U/L) | Total |
|---|
| Low | Normal | High |
|---|
| Case | LDL mg/dl | <100 | – | – | 10 | 10 |
| >100 | – | – | 20 | 20 |
| Control | LDL mg/dl | <100 | 4 | 15 | 6 | 25 |
| >100 | 0 | 4 | 1 | 5 |
Discussion
In our study 22/30 cases were in the age group of 36 to 45 years. Out of 30 cases, 5 were in the age group of 36-55 years, 3 cases were having age <35 years. Therefore, maximum cases (25/30) had age <45 years which proves the occurrence of CAD in relatively younger age in India.
The presence of high Total Cholesterol, TG and LDL-C and low level of HDL-C in premature CAD patients is consistent with the earlier studies [15]. Many studies have proved that high LDL-C, high TG and low HDL-C levels are associated with increased risk for the development of CAD [16–19]. Studies have shown that there is an increase in risk of coronary artery disease in patients with increased serum TG levels. Decreased levels of HDL along with increased levels of triglycerides together increase the risk of coronary artery disease. Many studies have shown that increase in the HDL levels and decrease in the triglycerides level lead to improvement in the outcome of the coronary artery disease.
But conventional lipid parameters fail to explain the occurrence of CAD in many patients. In our study also TC is <200mg/dl in 20 out of 30 cases (66.66%). LDL-C is <100 mg/dl in 10 cases out of 30 (33.33%). HDL-C is normal in 33% of the cases. The above findings point that nonconventional or advanced lipid parameters are involved in the pathogenesis of CAD and determination of parameters can explain the occurrence of CAD even in cases with normal or low LDL-C. oxLDL and sdLDL are two such parameters which can explain the occurrence of CAD in such patients.
Low-density lipoprotein molecules are not uniform in size and weight. They show variation in their size, weight and lipid composition. They are divided into two categories: large buoyant fraction and small dense fraction. According to some cross-sectional and prospective epidemiological studies; individuals with SdLDL particles have a higher risk for coronary artery disease than subjects with large, buoyant LDL particles [20–22]. Mechanisms mediating this increased atherogenecity of Sd LDL particles include increased oxidation, decreased binding to LDL receptor and increased binding of sd LDL to arterial wall [23]. Small LDL particle size also associates with other atherogenic changes in lipids and lipoproteins, especially elevated serum triglyceride, decreased HDL cholesterol concentrations and insulin resistance. In the 13-year follow-up of the Quebec Cardiovascular Study the Laval University group reported that small dense LDL is a strong and independent predictor of CAD [24]. Moon JY et al., have shown that high level of small dense LDL is associated with severity of CAD [25]. Our study has shown similar results that are in accordance with the studies done so far.
LDL-C undergoes oxidative modifications and is converted to ox-LDL which is highly atherogenic. Atherogenecity of ox-LDL is due to alteration in its biological properties due to oxidative modification of LDL, resulting in increased chemotaxis, more retention in subendothelial cells, modulation of growth factors and cytokine production from endothelial cells, smooth muscle cells, and macrophages [26]. Some studies have demonstrated elevated levels of circulating ox LDL in association with coronary artery disease [27]. Holvoet demonstrated the role of ox LDL in rapidly progressing coronary atherosclerosis [28]. Steinberg showed the how low density lipoprotein oxidation occurs and its pathological significance [29]. AIR Study explains the role of ox LDL in subclinical atherosclerosis and increased inflammatory cytokines [30–31]. In the middle-aged persons, receiver operating characteristic-curve analysis revealed that ox LDL had a higher sensitivity for coronary artery disease than did LDL-C and TC to HDL-C ratio. Study conducted by Augsburg have shown that oxidized LDL level was significantly higher in coronary artery disease patients as compared to the controls.
Two third patients in our study had normal LDL-C levels which cannot explain the risk of atherosclerosis in these patients. Small dense fraction of LDL has higher tendency to get oxidized LDL which enhance pro-inflammatory genes resulting in recruitment of monocytes into the sub endothelial space. Oxidized LDL is taken up by monocytes leading to the formation of foam cells which are basis of atherosclerosis. Lipid profile is almost same in most patients of CAD and fails to explain the high morbidity and mortality of CAD. Conventional lipid parameters are also unable to explain the premature onset, higher prevalence and severity of coronary disease in Indian population. But levels of small dense LDL and ox LDL not only can explain higher incidence and prevalence of CAD but also the higher morbidity and mortality as it depends upon depend upon the number of foam cells which depends on level of ox LDL.
Apolipoprotein A-1 and Apolipoprotein B are important markers of CAD [32–35]. Apo B and Apo A-1 are better discriminators of CAD than LDL-C and HDL-C. Low ApoB/Apo A-1 ratio is a better marker of premature CAD. APO A-1 is the major protein of HDL-C. Apo A-I is anti-atherogenic because of Role in reverse cholesterol transport, activation of the enzyme LCAT and prevention of the oxidation of LDL particles. Apo B is the chief protein component constituent of the atherogenic LDL-C, each LDL particle including one ApoB molecule. Hence, plasma ApoB levels reflect the total numbers of atherogenic particles.
Apo B/Apo A1 ratio is better marker of risk of CAD as compared to LDL-C and HDL-C. Lamarche B et al., showed that plasma ApoB concentrations showed a strong association with onset of IHD (risk ratio 1.4) independent of triglycerides, HDL cholesterol, and TC/HDL cholesterol ratio. Lamarche B et al., again explained that level of Apo B was higher in cases (130±33mg/dl) as compared to controls (110±27mg/dl) [35]. AMORIS study done in 2001 showed, Apo B/Apo A1 ratio as and predictor of fatal myocardial infarction [34]. Our study also show higher value of ApoB and lower value of ApoA1 in cases as compared to controls and the Apo B/Apo A1 ratio is low in cases. Results are in accordance with the values found in earlier studies. Apo A1 if lower in 20/30 cases and 5/30 controls. Apo B is higher in 28/30 cases and 8/30 controls.
Lp(a) is a genetically determined powerful and independent risk factor for macrovascular diseases due to atherosclerosis, including coronary artery disease, stroke and peripheral artery diseases. A high level of Lp(a) (> 30 mg/dl) increases the risk of premature CAD. The risk is increased several-fold in the presence of high levels of other lipid and non-lipid risk factors. Lp(a) was found to be significantly higher in young (<45 years of age ) patients with MI and their first degree relatives compared to controls which is proved by our study. Gupta et al., and Geethanjali et al., have observed significantly higher levels of Lp(a) in patients with CAD compared to controls [36,37]. Isser et al., showed that the Lp (a) level (22.28±5.4 mg/dl) was higher in patients as compared to controls (9.28±22.59 mg/dl) [38]. In addition, it was significantly higher in young patients with myocardial infarction as compared to controls. In our study Lp(a) levels are significantly higher in premature CAD patients as compared to controls. The values of Lp(a) is high in all the thirty cases and high in only three controls out of thirty.
Therefore alterations in the values of Oxidized LDL, Small Dense LDL, Apolipoprotein A-1, Apolipoprotein-B and Lipoprotein (a) when taken together in premature CAD, remain largely unexplored in India. Hence this study was undertaken to find the values and correlation of these markers in premature CAD which is assuming alarming proportions in India. Advanced lipid parameters are better determinants of premature CAD in Indians as compared to conventional lipid parameters according to our study. These parameters are especially more important in Indian population a prevalence of CAD is high and genetically influenced. oxLDL, Apo A1, ApoB, Lp(a) and sdLDL are genetically determined lipid parameters and better markers to explain pathogenesis of atherosclerosis. These markers are able to explain risk of CAD even in patients who have almost normal values of conventional lipid parameters. Estimation of these parameters may prove to be better for screening and early diagnosis of CAD so that corrective measures in the form of dietary modifications or drug therapy can be taken. These markers can also prove to be target for drug therapy.
Limitation
The main limitation of this study is the relatively small sample size (30 cases and 30 controls in our study). This study should be carried out by taking larger sample size so that these results can be validated and advanced lipid parameters can be utilized as the markers of premature CAD in addition to conventionally used ones.
Conclusion
oxLDL and small dense LDL explain the occurrence of coronary events in patients who have normal levels of LDL-C. The level of ox LDL, sdLDL and lipoprotein (a) can explain the higher occurrence of CAD in Indian population as these are genetically mediated and higher in Indians as compared to their western counterparts. The levels of oxidized LDL are responsible for the formation of foam cells which are marker of the severity of coronary events. Therefore levels of oxidized LDL, small dense LDL and lipoprotein (a) helps in early detection and intervention in premature CAD patients in India. They are also focus of targeted drug therapy in future as control of the levels of these markers will help in prevention of acute coronary events in Indian population.
These findings should be further explored in larger studies before implementation of advanced lipid parameters as better markers of CAD.
Where oxLDL= oxidized low density lipoprotein, Lp(a)= lipoprotein(a), Apo A-1 apolipoprotein A-1
ApoB apolipoprotein B-100, sd LDL= small dense low density lipoprotein
TG= triglyrides, HDL-C=high density lipoprotein-cholesterol, LDL-C= low density lipoprotein-cholesterol