Single embryo transfer (SET) is becoming the preferred mode of embryo transfer in many assisted reproductive centres. There is an intense focus on the prognostic biomarkers of embryo viability to select the best quality embryo for transfer. Perifollicular vascularity has been recognised as one among the many biomarkers available.
The quality of an embryo depends on the parent oocyte from which it is derived. Indeed, the developmental competence of an oocyte is dictated by the blood flow around the pre-ovulatory follicle in which it is developing, referred to as the ‘Perifollicular Vascularity’ (PFV) [1]. PFV closely reflects the intrafollicular environmental conditions, where well vascularised follicles have high dissolved Oxygen content and higher concentrations of angiogenic factors-Vascular endothelial growth factor, compared to the under-vascularised follicles [2,3]. Good PFV and favourable intrafollicular conditions during the preovulatory stage are vital for the final maturation of the oocyte and completion of the second meiotic division. Only mature oocytes from well vascularised follicles, upon fertilisation can produce embryos with better quality and implantation potential. Oocytes retrieved from poorly vascularized follicles exhibited higher frequencies of abnormal cytoplasmic organization, chromosomal misalignment and metaphase spindle defects. Such oocytes, after fertilization would produce embryos with chromosomal defects and result in early developmental arrest [2]. This was further justified by Mikkelsen et al., who showed that best grade embryos were developed from healthy oocytes while oocytes with asynchronous nuclear and cytoplasmic maturation produced poor quality embryos with impaired implantation potential [4]. Such embryos have higher chances of aneuploidies and chromosomal mosaicism that would result in early pregnancy loss.
Hence, it is inferred that the PFV correlates well with the follicular oxygenation, oocyte maturation, embryo viability and their implantation potential. PFV is amenable to imaging with use of Doppler Ultrasound. Power Doppler is a promising tool, which images the intensity and amplitude of a signal. It is the most sensitive mode for semi-quantitative measurement of low velocity blood flow and provides the best delineation of flow in microvasculature [5]. It is simple, safe, reproducible and acceptable to be used in the daily clinical setting.
To study the association of the PFV with the quality of the retrieved oocytes and embryos. To compare the same between the agonist and antagonist protocol employed in Assisted Reproductive techniques (ART).
Materials and Methods
A prospective observational study was carried out at the Reproduction wing of a tertiary care centre between September 2012 and September 2013. A total of 80 patients who underwent ART were enrolled in the study. A written informed consent was obtained from the subjects. The study was approved by the Institutional Ethics Committee. The patients were subjected to the standard protocol and infertility work-up practised at reproductive centre. Thirty patients received agonist protocol and 50 had received the antagonist protocol, the choice decided by the individual patient profile and controlled ovarian stimulation guidelines.
In the agonist protocol group, the stimulation protocol involved pituitary down-regulation with gonadotropin releasing hormone (GnRH) agonist from day 21 of the previous cycle, followed by gonadotropin stimulation from day 2 after ensuring adequate down-regulation (Serum estradiol < 30pg/ml, Endometrial thickness <4 mm).
In the antagonist protocol group, the stimulation protocol started with administration of gonadotropins- recombinant follicle stimulating hormone (FSH) from day 2 of cycle. Gonadotropin releasing hormone antagonist was started when any one of the following criteria was met: leading follicle was 12-14 mm or > 6 follicles of 11 mm or serum oestradiol > 400 ρg/mL. GnRH antagonist was continued upto the day of Inj. hCG.
In both the groups, the follicular development was monitored from day 6. Gonadotropins were continued till a day before the administration of Inj. hCG. Follicular maturation was triggered by administration of Inj. human chorionic gonadotropin (hCG) when at least 3 follicles acquired a size of 18 mm. Thirty-five hours later, follicular imaging was performed with Ultrasound (GE Logic E), equipped with 7.5 MHz Transvaginal curvilinear probe in Power Doppler mode. The imaging was done by the same operator to avoid inter-observer bias. During ultrasound scanning of each ovary, the follicle was first visualised in B- mode, then the power Doppler colour box was positioned over each ovarian follicle. Caution was exercised to ensure that signals were picked up from the follicle under study and not from the adjacent follicle or ovarian stroma. The cross-sectional image of the follicle with the maximum colour indication was frozen and then the PFV was graded, as depicted in [Table/Fig-1]. The semiquantitative grading of Perifollicular Vascularity (PFV), proposed by Chui et al., and Bhal et al., was used, which are as follows [6,7]:
Transvaginal ultrasound images of different grades of Perifollicular vascularity (PFV)
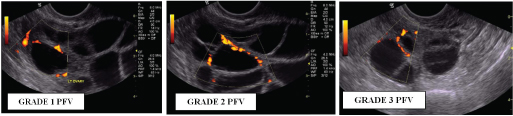
Grade 1: with vascularity ≤25% of follicular circumference;
Grade 2: with vascularity between 26 and 50% of follicular circumference.
Grade 3: with vascularity between 51 and 75% of follicular circumference.
Grade 4: with vascularity >75% of follicular circumference.
Oocyte pickup was done from all follicles ≥ 18 mm under ultrasound guidance. Each oocyte was collected in a separate tube and carried the same label of its parent follicle. The Embryologist was blinded and the subsequent processing, invitro fertilisation and culturing of embryos were carried out as per the standard guidelines. The oocyte and embryo quality were assessed and graded by the international guidelines and noted.
The following scheme of classification is considered for grading of the retrieved oocytes [8].
Immature
Prophase I: Germinal vesicle present; usually not inseminated or injected.
Metaphase I: No first polar body is present, no germinal vesicle; inseminated or injected 1-5 hours after extrusion of the first polar body.
Mature
Metaphase II: First polar body is present, no germinal vesicle; inseminated or injected 3-5 hours after collection.
Embryos are graded as per the by Consensus scoring system put forth by the European Society of Human Reproduction and Embryology (ESHRE) for cleavage-stage embryos, from the best to lowest grade [9]:
Grade 1: Lesser than 10% fragmentation and no multinucleation.
Grade 2: Between 10 and 25% fragmentation and no multinucleation.
Grade 3: Greater than 25% fragmentation and evidence of multinucleation.
Grade 1 and 2 embryos are considered good grade embryos while grade 3 embryos are considered poor grade.
The data including the age, body mass index (BMI), cause and duration of Infertility, basal hormonal profile, number of follicles, PFV grade of the follicles on the day of Inj. hCG, number of follicles tapped and the number of oocytes retrieved and embryos were obtained. The yield of oocytes and embryos were calculated by dividing the total number of oocytes and embryos respectively by the total number of follicles in each patient and expressed in percentages. The data were analysed with respect to perifollicular vascularity.
Statistical Analysis
Results were expressed in mean and percentages. The statistical significance was carried out with the application of Scientific Package for Social Sciences (SPSS version 16.0). Categorical variables were analyzed using the Chi-square (χ2) test. A one-way analysis of Variance (ANOVA-univariate) was applied to assess the mean values (as data were assumed to be normally distributed) of the different variables with respect to three groups (Grade 1, 2, 3 PFV). A Bonferroni correction was also applied to ascertain specific differences between these groups. A p-value < 0.05 was considered statistically significant.
Results
A total of 80 patients were recruited for the study, out of which 5 patients were excluded from the study (ovaries were inaccessible for oocyte retrieval in 3 patients and oocyte pickup was not done; cycle was cancelled in 2 patients in anticipation of ovarian Hyperstimulation syndrome). Hence, 75 patients were studied: 50 in the Antagonist protocol group; 25 in the Agonist protocol group.
The patient characteristics and profiles are presented in [Table/Fig-2]. There were no significant differences in the age, BMI, duration of infertility, among the subjects in the Agonist Protocol and Antagonist Protocol groups.
Patient profile and characteristics.
| Variable | Agonist group(n=25) | Antagonistgroup (n=50) | p-value |
|---|
| Age in years (mean ± SD) | 30.36 ± 3.8 | 32.04 ± 4.4 | 0.3 |
| BMI in kg/m2 (mean ± SD) | 27.65 ± 4.1 | 27.84 ± 2.8 | 0.8 |
| Type of infertility number (percentage) | Primary | 19(76%) | 35(70%) | - |
| Secondary | 6(24%) | 15(30%) |
| Duration of infertility in years (mean ± SD) | 5.76 ± 2.3 | 6.61 ± 2.9 | 0.2 |
| D2 FSH (mean ± SD) | 5.53 ± 1.7 | 6.56 ± 2.5 | 0.5 |
| D2 LH (mean ± S.D) | 6.17 ± 5.8 | 4.96 ± 2.3 | 0.2 |
BMI: Body mass index; FSH- Follicle stimulating hormone; LH- Luteinising hormone
The aetiology of infertility in Agonist and Antagonist group were as follows: Male factor - 28% and 37%, Polycystic ovarian syndrome - 24% and 4%, Endometriosis- 16% & 10%, Tubal factor – 8% & 25%, Unexplained – 20% and 20% and Both partners – 4% & 2% respectively.
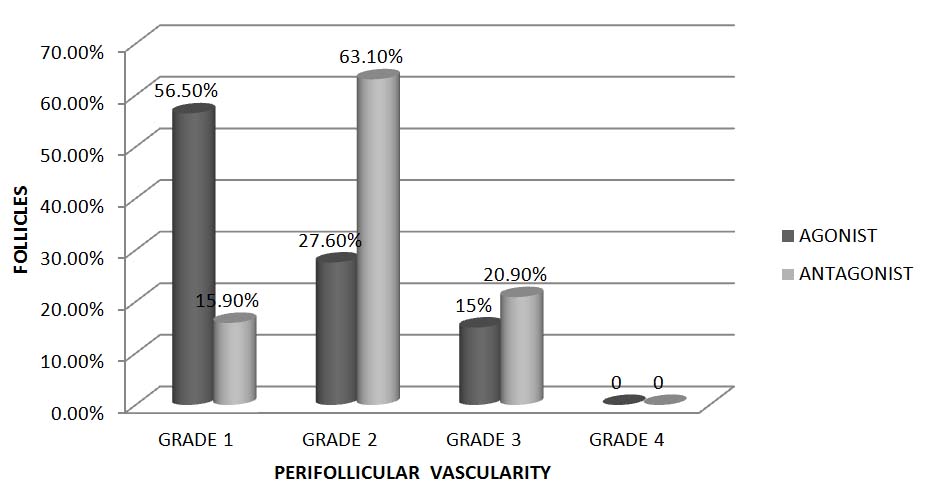
The PFV of a total of 1245 follicles among 75 subjects was studied. The distribution of follicles with different grades of vascularity in both the groups is depicted in [Table/Fig-3]. The Agonist and Antagonist group had predominantly follicles with grade 1 PFV and grade 2 PFV respectively. Follicles with grade 3 PFV were similar in both the groups. Follicles with grade 4 PFV were not observed in the current study.
Distribution of follicles with different grades of PFV, expressed in percentage
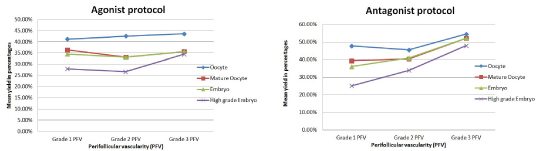
On the day of oocyte retrieval, the yield of oocytes with respect to PFV was noted [Table/Fig-4]. Oocyte retrieval showed an increasing trend with respect to increasing vascularity in both the groups, but was not statistically significant.
The yield of oocytes with respect to PFV, expressed as percentage (mean±SD)
| Grade 1 PFV | Grade 2 PFV | Grade 3 PFV |
|---|
| Agonist group (n=25) | 41.27(± 19.09) | 42.62 (± 14.63) | 43.63 (± 13.59) |
| Antagonist group (n=50) | 47.82(± 28.11) | 45.55 (± 12.47) | 54.52 (± 17.57) |
p-value: 0.617 (PFV grade); 0.129 (group)
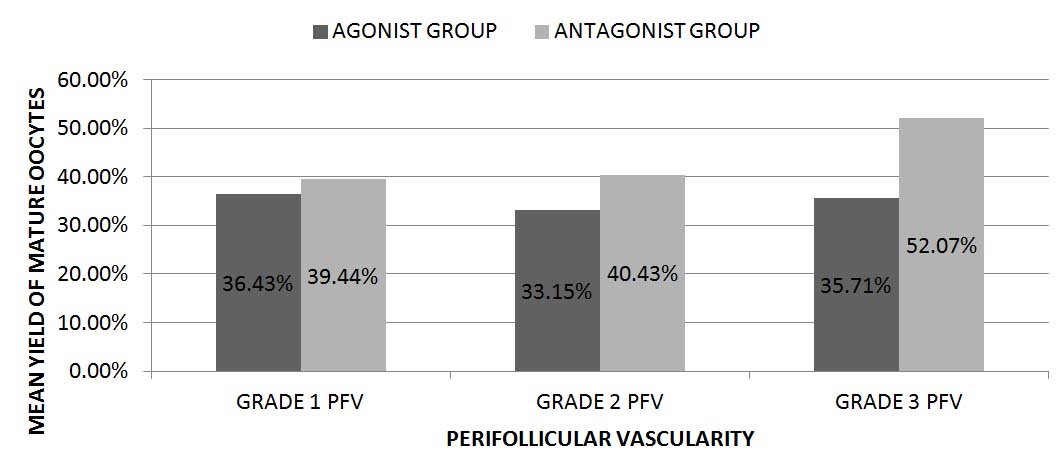
The yield of mature oocytes, even though were independent of the perifollicular vascularity, was found to be better and statistically significant (p=0.03) in the Antagonist group compared to the Agonist group and is represented in [Table/Fig-5].
The mean yield of mature oocytes with respect to Perifollicular vascularity, expressed in percentage
p-value: 0.35(PFV); 0.03 (group)
The yield of embryos with increasing PFV was statistically significant (p=0.04) in the Antagonist group, illustrated in [Table/Fig-6].
The yield of embryos with respect to PFV, expressed as percentage (mean±SD)
p-value: 0.23 (PFV grade); 0.04 (group)
| Grade 1 PFV | Grade 2 PFV | Grade 3 PFV |
|---|
| Agonist group (n=25) | 34.58 (± 18.38) | 33.15 (± 13.49) | 35.71 (± 12.75) |
| Antagonist group (n=50) | 36.1 (± 22.05) | 40.87 (± 13.14) | 52.07 (± 17.88) |
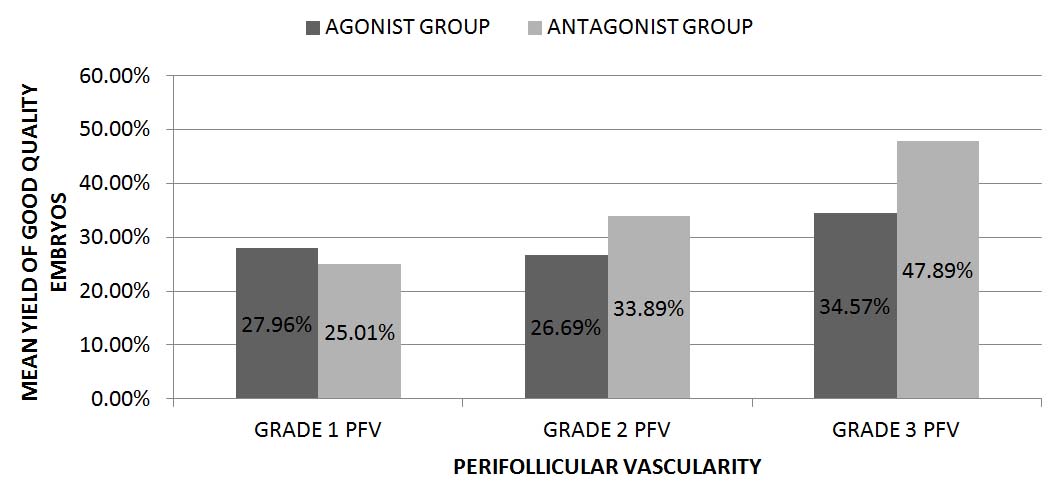
The mean yield of good quality embryos was observed in both the groups [Table/Fig-7] in conjunction with the PFV of the parent follicle and was found to be highly significant (p=0.003). No statistically significant difference was observed in between the groups (p=0.07). Sub-analysis observations have shown that follicles with grade 3 PFV yielded higher percentage of good quality embryos than follicles with grade 1 & 2 PFV; p=.003 (Grade 1 versus grade 3 PFV); 0.02 (Grade 3 versus grade 2 PFV).
The mean yield of good quality embryos with respect to Perifollicular vascularity, expressed in percentage
p-value: 0.003 (PFV grade); 0.07 (group)
Discussion
Previous studies had employed the Power Doppler to assess the PFV [6,7,10–12]. They have observed association between the PFV and various parameters such as, oocyte retrieval, maturity of oocytes, fertilization rates, triploidy rates and pregnancy rates. In the present study, all the above mentioned associations have been elucidated separately in GnRH agonist and GnRH antagonist regimens. In the available literature searched, the current study was the first one to do so.
Studies by Chui et al., Bhal et al., and Palomba et al., have observed, majority of follicles with high grade PFV (Grade 3 & 4 PFV) [6,7,12]. The observation of the distribution of follicles with different grades of PFV in the current study is contradictory to that made in the previous studies. The current study had included subjects with Polycystic ovaries and endometriosis, which could partly explain their unfavourable influences on the PFV and hence the deviation from the observations of the other studies.
With respect to the association between PFV and oocyte retrieval, most consistent results were shown by Bhal et al., where the oocyte retrieval and the yield of mature oocytes strongly correlated with the PFV of follicles (p=0.05), suggesting that oocyte attains better maturity with increasing PFV of the parent follicle [7]. Other studies by Chui et al., and Robson et al., found the yield and maturity of oocytes to be independent of the PFV [6,10]. In all the above mentioned studies, there was no group stratification as all patients were subjected to IVF- agonist protocol. The present study under discussion did not find statistically significant association between PFV of follicles and oocyte retrieval in both the groups. But in the antagonist group, there was a better yield of mature oocytes compared to the agonist group. A better PFV grade in the antagonist group could be the most probable explanation.
The percentage yield of good embryos from parent follicles with high grade PFV were 66.7% and 56.7% in the previous studies by Chui et al., and Bhal et al., respectively [6,7]. The yield was much lesser from follicles with low grade PFV but however, the difference was not statistically significant. In the present study, apparently the yield of embryos was becoming better with PFV grade. But this difference was not statistically significant. While comparing in between the two groups, the yield of embryos were significantly higher in the antagonist group (p= 0.04), which could probably be explained by the better yield of mature oocytes. A study by Murber et al., had shown that GnRH antagonist had positive influences on early embryo development, thus resulting in good quality embryo rate [13]. The present study had shown a strong association between the PFV and the yield of good quality embryos (p=0.003). The highest percentage of good quality embryos were obtained from grade 3 PFV. The current study had supported authentically the observations made previously by Chui et al., and Bhal et al., [6,7]. Chui et al., had noted that the embryos derived from oocytes of poor vascular follicles resulted in statistically significant higher triploidy rates when compared with those derived from good vascular ones [6]. The current study did not evaluate the relationship between the PFV and triploidy rates.
Pregnancy rates were observed to be 0%, 22% and 42% in the Agonist group and 17.4%, 69.6% and 13% in the Antagonist group with reference to PFV grade. The conclusions derived from other studies are as follows: Chui et al., had reported that there were no pregnancies in that group of women, whose embryos were derived from poor vascular follicles [6]. The study by Bhal et al., Borini et al., and Robson et al., produced similar results [7,11,10]. Pregnancy rates were significantly higher in the group with high Perifollicular vascularisation than in the group with low vascularisation. The study by Palomba et al., produced contradictory results and pregnancy rates had no correlation to the PFV, as observed in the current study [12]. Pregnancy depends on other parameters besides PFV, such as the sperm quality, endometrial receptivity etc. Since these factors were not taken into consideration, pregnancy cannot be reliably predicted. However, in the present study, pregnancy rates were the highest in patients with grade 3 PFV in the Agonist protocol group.
Limitation
The present study is not without flaws. The following are the drawbacks: The patients in the two groups chosen were not age-matched and BMI-matched which could have confounded the results. The cause of infertility was not homogenous in both groups. The dosage of Gonadotropins used for Controlled Ovarian Hyperstimulation was not fixed in both the groups and were modified according to the folliculogenesis. Semen parameters were not taken into consideration which can also affect the embryo quality. PFV alone cannot predict the implantation potential of the embryo, which is a key factor for successful outcome.
A summary of the association between PFV and the mean yield of oocytes and embryos is presented in [Table/Fig-8a,b].
Comparison between Agonist and Antagonist protocol in terms of the yield of oocytes and embryos with perifollicular vascularity
Conclusion
Antagonist protocol had many favourable outcomes compared with the agonist protocol. The retrieval of oocytes, even the mature ones and the yield of high grade embryos were found higher in the antagonist protocol. As the PFV increased, the yield also increased. Predominant follicles had Grade 2 PFV. It was in this group of subjects that the overall pregnancy rates were the highest too.
It is practically feasible in clinical settings to determine the PFV of the follicles in stimulated ART cycles. Determination of PFV by Power Doppler can be incorporated as one of the parameters for deciding oocyte retrieval since high grade embryos were derived from follicles with higher PFV. Hence, PFV as assessed by Power Doppler is a useful non-invasive biomarker of embryo quality. Though the present study and previous literature do not warrant its routine implementation, it can be employed along with other biomarkers in ART to predict successful outcome.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
BMI: Body mass index; FSH- Follicle stimulating hormone; LH- Luteinising hormone
p-value: 0.617 (PFV grade); 0.129 (group)