Intestinal obstruction is the common surgical problem in neonates of which intestinal atresia and stenosis contributes one third of cases. Ratan et al., study found intestinal atresia is the second most common cause (11.8%) of intestinal obstruction in newborns after intussusception (20.8%) in all age groups [1]. Pathogenesis of intestinal atresia is still controversial. Stowens (1966) and Morrison (1970) proposed atresia and stenoses are caused by failure of recanalization of the bowel lumen following a developmental stage of obliterative epithelial proliferation [2]. In contrast, Louw and Barnard (1955) suggested an intrauterine vascular insult is responsible for atresia and stenosis [3]. Hence this study is undertaken to study the histopathological features of intestinal atresia and to correlate with clinical features.
The present study describes the clinical and histomorphological features in cases of congenital atresia and stenosis in 39 cases studied over a period of 5 years.
Materials and Methods
This is a retrospective study, studied over a period of 5 years, from 2007 to November 2011. The records of the Department of Pathology, Maulana Azad Medical College were searched for cases of intestinal obstruction of newborn infants. Out of total 147 cases, those cases where intestinal obstruction was due to intussusception, volvulus, meconium ileus, and Hirschprung’s disease were excluded from the study. Thirty nine cases of intestinal atresia were traced out, the older slides were reassessed and fresh sections cut. Special stains like masson trichrome, von kossa and perl’s staining were done to demonstrate fibrosis, calcification and haemorrhage. Details of age, sex, birth weight, age at symptoms presented and associated malformations were noted. These cases were classified using Martin & Zerella’s [4] and Louw’s classification [5], as: Type I: Septal Atresia. Type II: Fibrous cord joining atretic ends. Type IIIa (Louw’s): Atretic ends separated by V shaped mesenteric defect. Type IIIb: Multiple atresias Type IV: Apple peel Atresia.
Results
Out of 39 cases of intestinal atresia 26 were males and 13 were females with male: female ratio of 2:1. Cases involving ileum only [Table/Fig-1] (23 cases-59%), jejunum only (12 cases – 30%) and ileum and jejunum, duodenum, ascending colon [Table/Fig-2], sigmoid colon (1 case each). All of them presented within first week of life, with the clinical features of intestinal obstruction like bilious vomiting, not passing meconium since birth, peristalsis etc. The mean age at presentation was 2.2 days, except two cases- one case presented on 12th day was associated with gangrene, perforation & mucormycosis, while the other presented on 25th day which was associated with perforation as a complication. Complications included gangrene (4 cases), perforation (5 cases) and meconium peritonitis (2 cases), fungal infection (mucormycosis) in one case. These cases were classified into type I (4 cases -10%), type II (24 cases – 59%), type IIIa (1 case –2.5%), IIIb (3 cases 7.5%) and type IV (5 cases 12.5 %). In 2 cases typing could not be done due to lack of details.
Gross photograph of ileal atresia
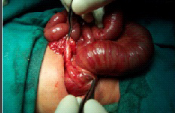
Peroperative picture colonic atresia
Associated anomalies [Table/Fig-3] and Histological features [Table/Fig-4] shown in Tables.
| Associated anomalies | No of cases | Site of atresia |
|---|
| Gastroschisis (GS) | 1 | Ileum |
| Volvulus | 1 | Ileum |
| Anal stenosis | 1 | Ileum |
| Microcolon | 1 | Ileum |
| Annular pancreas | 1 | Proximal jejunum |
| Meconium cyst | 1 | Ileum |
| Duplication cyst | 3 | Jejunum in 1 case, ileum in 2 cases |
| Mucosa(No. of cases) | Submucosa(No. of cases) | Muscularis propria(No. of cases) | Serosa(No. of cases) |
|---|
| Oedema, congestion & denudation (11) | Oedema (7) | Vascular proliferation (8) | Congestion (11) |
| Ulceration & flattening of mucosa (14) | Congestion (5) | Fibrosis (7) | Fibrosis (5) |
| Abnormal villus configuration [Table/Fig-5](2) | Dilated irregular branching vascular channels (23) | Thickening (4) and thinning (2) | Serositis & serosal exudates (8) |
| Gangrene (4) | Mesenchymal condensation around the blood vessels [Table/Fig-6] (8) | Segmental absence of muscularis propria (Table/Fig-7) (2) | Prominent vascular proliferation (3) |
| Complete luminal obliteration (3) | Haemangiomatous proliferation of vessels (18) | | |
| Luminal narrowing (2) | Fibrosis [Table/Fig-8] (29) | | |
| Squames in the lumen [Table/Fig-9] and lanugo hair distal to obstruction (2) | Submucosal haemorrhage and haemosiderin laden macrophages [Table/Fig-10] (2) | | |
| Calcification (6) [Table/Fig-11] | | | |
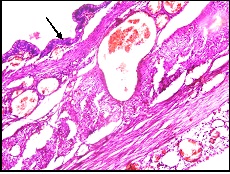
Flattening of villous (arrow) and abnormal villous configuration (H&E Medium magnification 20X)
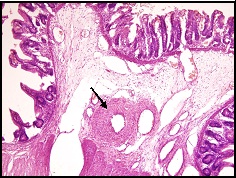
Mesenchymal condensation around the blood vessels (H&E Scanner view 4X)
Mucosal ulceration, calcification (black arrow) and segmental absence of muscularis propria (red arrow) (H&E Low magnification 10X)
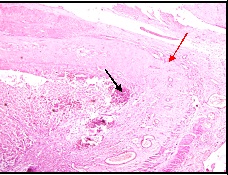
Masson trichrome stain highlighting the submucosal fibrosis (H&E Medium magnification 20X)
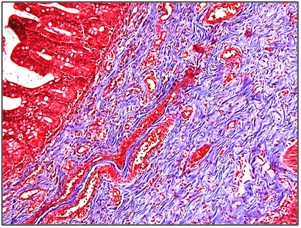
Squames (arrow) in the lumen distal to the obstruction (H&E Low magnification 10X)
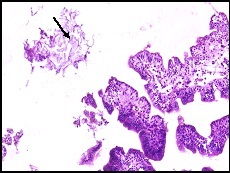
Haemosiderin laden macrophages (arrow) in the submucosa indicating old haemorrhage (H&E high magnification 40x)
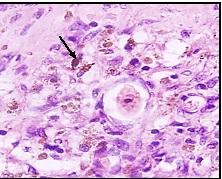
Von kossa stain highlighting the calcification (H&E Low magnification 10X)
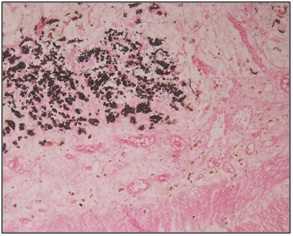
Full thickness gangrene was seen in 4 cases, out of which one was associated with fungal infection (mucormycosis) [Table/Fig-12 and 13]. Five cases were associated with perforation, out of which 4 showed foreign body giant cell reaction in the wall.
Necrotic tissue with mucormycosis (arrows) (H&E) (H&E Medium magnification 20X)
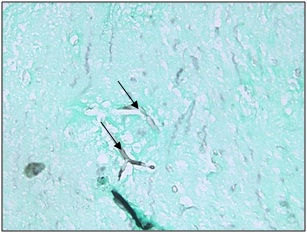
Silver Methanamine stain showing mucor (H&E Medium magnification 20X). (arrow)
Discussion
Intestinal atresias are one of the most common causes of intestinal obstruction in the neonate with an incidence of 1 in 5,000 newborns [6]. In the present study out of 147 cases of neonatal intestinal obstruction, 39 cases were due to atresia which accounts for one third cases of neonatal intestinal obstruction as observed in various other studies. Small intestinal atresia accounts for majority of the cases while colonic atresias are quite rare [7]. Four types of intestinal atresia are recognized. Septal atresia in which the bowel is continuous, the blind end in which bowel is not in continuity, and the cord like segments, which may separate the intestine in multiple segments in case of multiple atresias, associated mesenteric defect and apple peel atresia. The most common is type 2 atresia in our study about 59%, the incidence of multiple atresia is said to be 15% [8], in our case series it was seen in 3 cases (7.5%). Ileal atresia is the most common site noted in our study.
Gastroschisis (GS) complicated by intestinal atresia has been described in the literature. GS can be described as simple, in cases of isolated GS, or complex, where there is an associated atresia, perforation, necrotic segment or volvulus. An atresia in association with GS occurs in 5–15% of patients and is a poor prognostic indicator [9]. In our study the only case associated with gastroschisis was complicated by perforation and gangrene.
Various pathogenetic mechanisms have been proposed to explain the development of both duplication cysts and intestinal atresia. Favara et al., and Sinha et al., attributed the occurrence of atresia secondary to mesenteric volvulus as being initiated by the duplication cyst, leading to vascular compromise of the involved intestinal segment [10,11]. In our study 3 cases were associated with duplication cyst.
Vascular proliferation and haemangiomas complicating as intestinal atresia have been well documented in literature [12,13].
Segmental differentiation of gut starts as early as 3 weeks of gestation. Fetal bile secretion and the swallowing of amniotic fluid begin in at 11-12 weeks of gestation, well after recanalization of the solid cord stage [14]. The small intestinal epithelium in humans is mature at birth. Luminal components such as amniotic fluid (before birth) and enteral nutrition (after birth) are not essential for epithelial maturation. Rather, epithelial protein expression (genetically imprinted) is crucial for nutrient absorption, epithelial defense, and repair of the small intestine [15].
The aetiology of small intestinal atresia varies with its site of origin. Tandler, while investigating the embryology of the duodenum, postulated intestinal atresia to result from failure of recanalization of the intestinal lumen following obliteration during the stage of epithelial proliferation [16]. This phase of proliferation and recanalization occurs prior to the eleventh week of intrauterine life. Failure of recanalization seems the best explanation for localized duodenal atresia or stenosis. In jejuno-ileal atresia, however, bile, squamous cells and languo, all of which are produced after the stage of epithelial proliferation, may be found distal to the site of obstruction [4]. Since excretion of bile begins about the eleventh week and squamous epithelial cells swallowed from the vernix caseosa are found in the intestine after the twelfth week, the presence of these elements in the intestinal lumen distal to any level of congenital atresia would indicate that occlusion occurred sometime after the eleventh or twelfth week of fetal life, well beyond the period of development of the intestinal tube [4]. Furthermore, the segmental absence of the jejuno-ileal mesentery cannot be accounted for on the basis of a failure of recanalization. A more plausible explanation is that jejunoileal atresia is a consequence of an intra-uterine mesenteric vascular accident secondary to volvulus, incarceration in a prenatal umbilical hernia, or intussusceptions [16,17].
Louw and Barnard experimentally produced the entire spectrum of neonatal intestinal obstruction by ligating the mesenteric vasculature of fetal puppies [2,3]. More proximal the mesenteric vascular compromise greater the resulting severity of the intestinal atresia. Similar observations have been made by Abrams [18]. The extent of these changes depends upon the time of development of the obstruction as well as the site of involvement.
Intestinal atresia associated with in utero intussusception, volvulus, perforation and gastroschisis further corroborates a vascular accident as the aetiology most of jejuno ileal atresias rather than failure of recanalization [5,16,17,19]. Histological features in our study, gangrene, perforation [20] all suggest the spectrum of bowel ischemia coupled with features of repair and regeneration rather than failure of recanalization in case of jejuno ileal atresias. Intramural bowel calcification at birth is rare, and has always been reported in bowel atresia, meconium ileus, and intrauterine volvulus. These associations suggest an ischemic damage to the bowel wall is an important prerequisite for calcification [21]. The spectrum of histological features depends on the extent, severity and duration of an ischemic event. The mucosa and submucosa is more susceptible to ischemic injury than muscularis. Injury to full thickness of the bowel may result in meconium peritonitis and perforation. If the ischemic segment undergoes healing rather than perforation, there may be scarring of mucosa, submucosa and muscularis leading to stenosis or atresia [22].
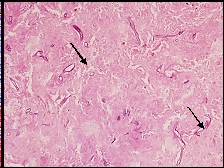
We found a peculiar feature of mesenchymal condensation around the blood vessels in 8 cases, this finding has not been described in literature in intestinal atresia, however similar feature is seen around ducts in dysplastic kidneys in which insulin like growth factor has been postulated in animal studies [23].
Rare anomaly like segmental absence of muscularis noted in our case is also associated with atresia. Segmental absence is most commonly found in the jejunum or ileum. The pathogenesis of congenital absence of muscularis propria is unknown. The causes proposed include abnormal embryogenesis leading to incomplete or discontinuous myogenesis, failure of mesenchymal condensation after regression of embryonic small intestinal diverticula, vascular accident in utero leading to inappropriate myogenesis [24,25]. Intrauterine intussusception is known to cause intestinal ischemia, with development of atresia and congenital absence of muscularis propria in premature neonates [26]. Segmental defects in muscular and neural structures of the intestinal wall observed in both the antimesenteric and mesenteric sides of the atretic small bowel were considered to support the vascular insult theory as an aetiologic factor [27] which explains the vascular compromise leading to segmental absence of muscle in atresia cases.
Thus the histological changes observed in the present study suggest that the affected infants were suffering from intrauterine intestinal ischemia contributing to the development of atresia. Further, mucosal changes seen on the stenotic segments are extremely similar to the persistent mucosal changes in the colon following the ischaemic phenomenon associated with Hirschsprung’s disease [28].
Moreover presence of lanugo, squames, and meconium distal to an atretic segment (Santulli and Blanc, 1961) [8] or segments of atretic bowel separated by relatively normal bowel and presence of meconium (Schultz and Lawrence, 1960) [29] explains ischemia is an aetiological factor rather than failure of recanalization. Further, a phase of obliterative epithelial proliferation is not seen in the small bowel below the duodenum (Johnson, 1910) [2] and it’s inappropriate that it was an explanation of stenosis/atresia of the small or large bowel.
Conclusion
Spectrum of histological features like mucosal edema, congestion, ulceration, flattening of villi, submucosal oedema, prominent vascular proliferation, fibrosis, haemorrhage, gangrene, calcification and the unusual finding of squames and lanugo hair distal to the atretic segment indicate that an in utero vascular accident that occurs relatively late in gestation (>11-12 weeks) is likely the origin of these atresias, rather than failure of GI tract recanalization. An intrauterine intestinal ischemia due to vascular pathology followed by resorption of the bowel is the possible explanation for the development of intestinal atresia.