Expression of Osteopontin in Oral Squamous Cell Carcinoma and its Surgical Margins-An Immunohistochemical Study
Vijaya Nirmala Subramani1, Malathi Narasimhan2, Muthukumar Thiyagarajan3, Balu David Munuswamy4, Logeswari Jayamani5
1 Senior Lecturer, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India.
2 Professor and Head, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai, India.
3 Associate Professor, Department of Anatomy, Sri Ramachandra University, Chennai, India.
4 Former Director, Government Arignar Anna Memorial Cancer Research Institute & Hospital, Kancheepuram, Tamilnadu, India.
5 Senior Lecturer, Department of Oral Pathology and Microbiology, Meenakshi Ammal Dental College, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vijaya Nirmala Subramani, No.17, Plot No:12/1,3rd Main Road, Lakshmi nagar, Porur, Chennai-600116, Tamil Nadu, India.
E-mail: subramani.viji3@gmail.com
Introduction
Despite the advances in the treatment modalities offered for oral squamous cell carcinoma. The recurrence rate of it still remains quite high. Early detection of recurrence will improve the outcome and the survival of the patient. Osteopontin, a transformation–related phosphorylated protein in epithelial cells has been closely related with tumourigenesis. This study was undertaken to explore the potential of OPN as a tumour marker of recurrence in OSCC.
Aim
To analyse the expression of Osteopontin (OPN) in Oral Squamous Cell Carcinoma (OSCC), patient matched tumour free surgical margins and normal oral mucosa and to correlate with local & loco regional recurrence.
Materials and Methods
Twenty cases each of formalin fixed paraffin embedded blocks of histopathologically diagnosed cases of OSCC, patient matched tumour free surgical margins and normal oral mucosal tissues were obtained from the archives of the Oral Pathology & Microbiology Department, Faculty of Dental Sciences, SRU and Govt. Arignar Anna Memorial Cancer Hospital, Kancheepuram. Immunohistochemical analysis was performed with an antibody to Osteopontin protein. Patients with secondary tumours and those treated with chemotherapy and radiotherapy were excluded from this study.
Results
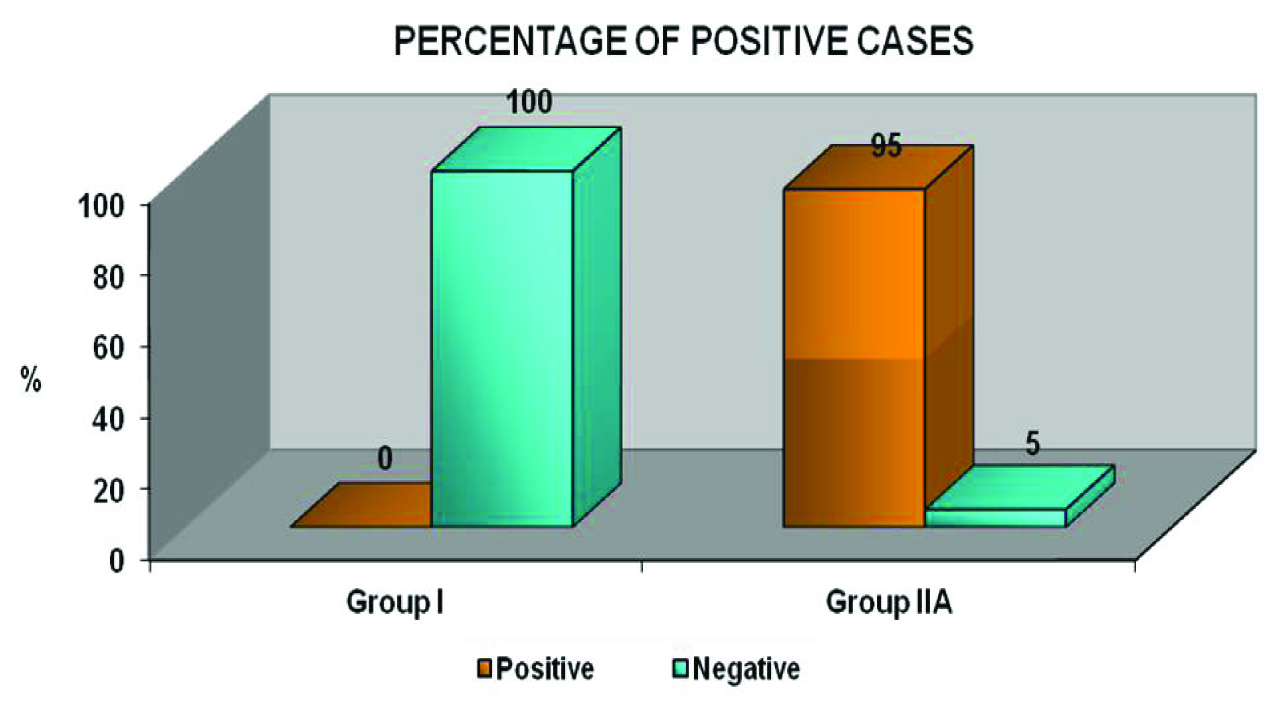
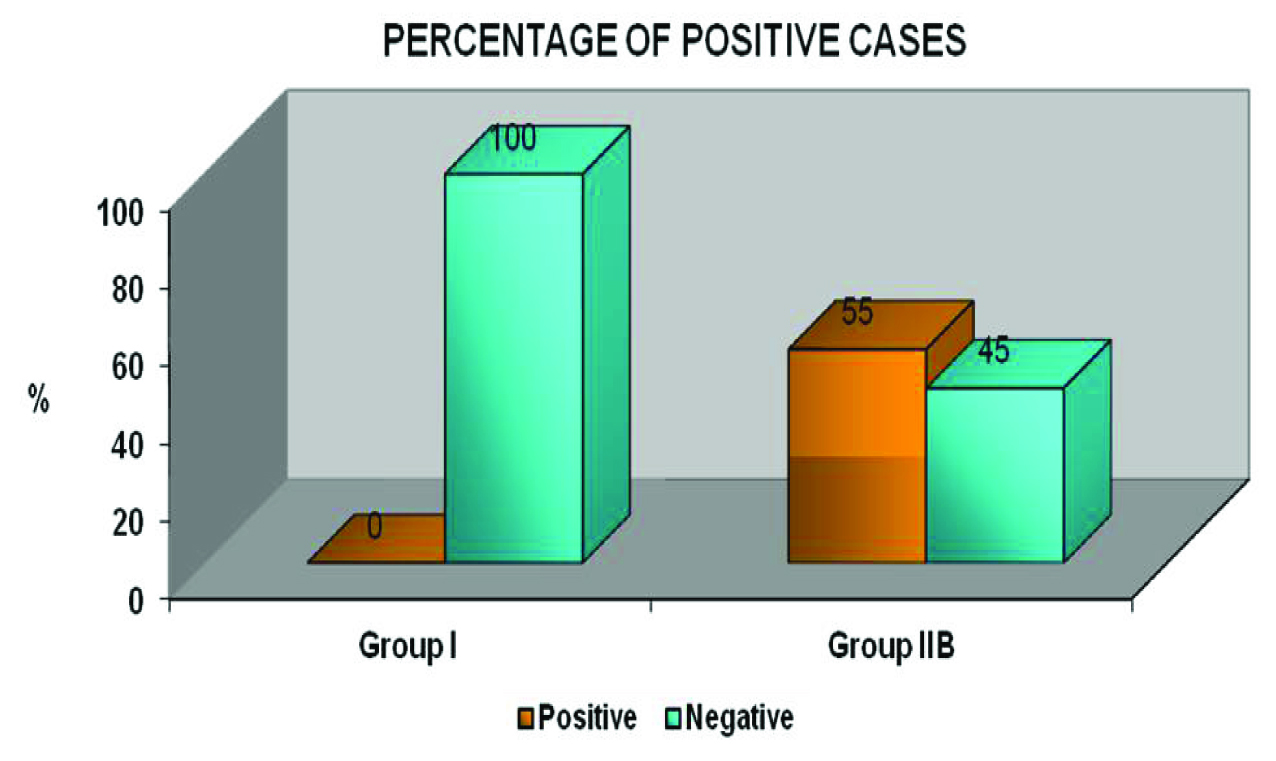
The expression of OPN was elevated in 95% of tumours & 55% of histologically tumour free margin samples. There was negative OPN expression in normal mucosal samples. The result of the study was statistically analysed using Pearson chi-square test and was found to be statistically significant.
Conclusion
OPN can be used as a diagnostic marker in Oral Squamous Cell Carcinoma. In the tumour free surgical margins, elevated levels of OPN may predict a significantly increased risk of recurrence.
Immunohistochemistry, Surgical margins, Recurrence, Tumourogenesis
Introduction
Head & neck squamous cell carcinoma is the fifth most common malignant neoplasm which arises from diverse anatomical locations including oral cavity, oropharynx, larynx & hypopharynx [1]. In India cancer registries have confirmed a high evidence of oral cancer due to widespread habits of tobacco chewing, smoking and alcohol consumption [2]. Human papilloma virus has also been recently implicated in the aetiopathogenesis of OSCC [2,3]. The mutagenic effects of tobacco, alcohol, betel quid or areca-nut are dependent upon dose, frequency and duration of use, and are accelerated and exaggerated by the concurrent use of two or more of these agents [4]. Owing to the lack of the clinical symptoms in its early stages, the diagnosis of oral cancer still remains a challenging task [5]. The recurrence rate & metastasis of tumour is 30% even though the surgical margins are diagnosed as tumour free [6].
Materials and Methods
A retrospective study was performed at the Department of Oral Pathology & Microbiology, Faculty of Dental Sciences, Sri Ramachandra University. The study samples consisted of 60 formalin fixed paraffin embedded biopsy specimens-20 Normal mucosa & 20 samples each of lesion and tumour free surgical margins from resected head and neck specimens. A tumour free surgical margin refers to the margins of the uninvolved tissue around the excised neoplasm which has been histopathologically confirmed.
The study samples were divided into:
Group I - Normal oral mucosa.
Group II A - Well differentiated Squamous cell carcinoma.
Group II B - Patient matched tumour free surgical margins.
A 5μm sections were cut and mounted on positively charged glass slides, deparaffinized with xylene and rehydrated in graded ethyl alcohol and water. To block endogenous peroxidase activity and non specific background staining, the sections were treated with peroxide block for 15 minutes at room temperature. After being washed with water for 5 minutes, antigen retrieval was done by pressure cooker method using citrate buffer solution at pH 6.0. Sections were stained with Rabbit Monoclonal Osteopontin Primary Antibody (ab91655 -EPR3688, Abcam, UK, 1:250) for 60 minutes at room temperature. After rinsing with Tris buffer for 5 minutes, slides were incubated with secondary antibody (ab80436, Abcam, UK) for 30 minutes at room temperature and washed with Tris buffer twice. Finally the sections were incubated with DAB- 3,3’diaminobenzidine and then rinsed in distilled water. All slides were counter stained with haematoxylin for 1 minute, washed in running water, dehydrated and mounted with DPX – Dibutyl phathalate xylene. Haematoxylin and Eosin sections of the representive cases were also prepared. Evaluation of immunostaining was performed by a pathologist who was blinded with the clinical details. Human colon carcinoma was used as a positive control for the IHC technique as per the instructions provided in the Immuno Histo Chemistry test kit instruction manual. The results of the study were statistically analysed by using Pearson chi-Square test.
Results
Homogenous dark brown cytoplasmic & membranous staining was taken as positive expression and significant OPN expression were observed in 95% (19/20) of Group II A [Table/Fig-1a–d] & 55% (11/20) of Group II B [Table/Fig-2a&b,c&d,e-g,h-j,k-l]. No OPN immunoreactivity was observed in Group I [Table/Fig-3a&b]. There were no significant differences among the various clinical parameters such as age, gender & site. A statistically highly significant difference in the expression of OPN was found between Group I, Group II A & II B (p-value=0.000) [Table/Fig-4a&b]. On the other hand, there was no statistically significant difference in expression of OPN in the intra study groups (Group II A & B).
(a) Squamous cell carcinoma with epithelial pearls (H&E-40X), (b) Strong OPN expression in the dysplastic epithelium and squamous cell carcinoma islands in the connective tissue (IHC-20X)
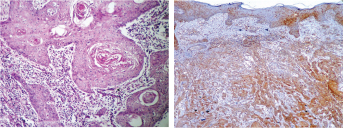
(c) Intense staining of OPN in peripheral cells of squamous cell carcinoma island (IHC-40X), (c) Intense OPN cytoplasmic and membrane expressions (IHC-40X)
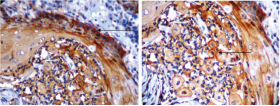
Positive OPN stain in epithelium in tumour free surgical margin (H&E, IHC-20X)

Positive staining of OPN in inflammatory cells and stromal cells in tumour free surgical margin (IHC-10X & 40X)

Lymph node with germinal center in tumour free surgical margin (H&E-10X, IHC-20X & 40X)

Positive OPN expression – muscle in tumour free surgical margin (H&E -20X, IHC-20X & 40X)

Positive OPN expression – salivary glands in tumour free surgical margin (H&E, IHC-20X)

Study Control -Normal epithelium Showing Negative OPN expression (H&E- 20X, IHC-20X)

Graph Bar diagram depicting the distribution of the IHC OPN staining expression of the study samples (group I & IIA)
Graph Bar diagram depicting the distribution of the IHC OPN staining expression of the study samples (group I & IIB)
Discussion
OPN, a secreted phosphorylated glycoprotein in the extracellular matrix was discovered by Senger et al., who defined it as a transformation related phosphorylated protein [7]. OPN is secreted by a variety of cells including osteoblasts, endothelial cells, epithelial cells, smooth muscle cells and macrophages. It is expressed in many kinds of cancers and has been associated with tumour cell growth, proliferation, invasion and metastatsis [8–12]. OPN promotes the development of malignancy by its stimulation of cellular transduction pathways through αvβ3 [13]. Increased expression of the β3 integrin has been correlated with the increased invasiveness in melanoma lines [14]. Petrik et al., suggested plasma OPN is an independent prognostic marker for head & neck cancer [15]. Two studies have been carried so far, to evaluate the expression of OPN in human OSCC [16,17]. To the best of knowledge, this is the first study to analyse the expression of OPN in OSCC and patient matched tumour free surgical margins of OSCC.
In this study, human colon carcinoma sections were used as the IHC Test control [Table/Fig-5a–c]. There was a negative expression of OPN in all the 20 samples of normal oral mucosa [Table/Fig-3a&b]. A similar expression was also seen in the studies conducted by Devoll et al., & Zhou et al., [18,19]. In OSCC samples (group II A), 95% of cytoplasmic & membrane immunoreactivity was observed in the tumour islands [Table/Fig-1a–d]. These results were in accordance with the findings of Devoll et al., who reported significant percentage of OPN expression in cell lines derived from OSCC [18]. However, one case of OSCC in our study showed negative immunoreactivity for OPN. This may be attributed to lack of accessibility of antigen for its specific antibody as validated by Rudland et al., [20].
Human Colon Carcinoma-Batch control (H&E-20X, IHC-10X)

Section shows Negative Batch control human colon carcinoma (IHC-20X)
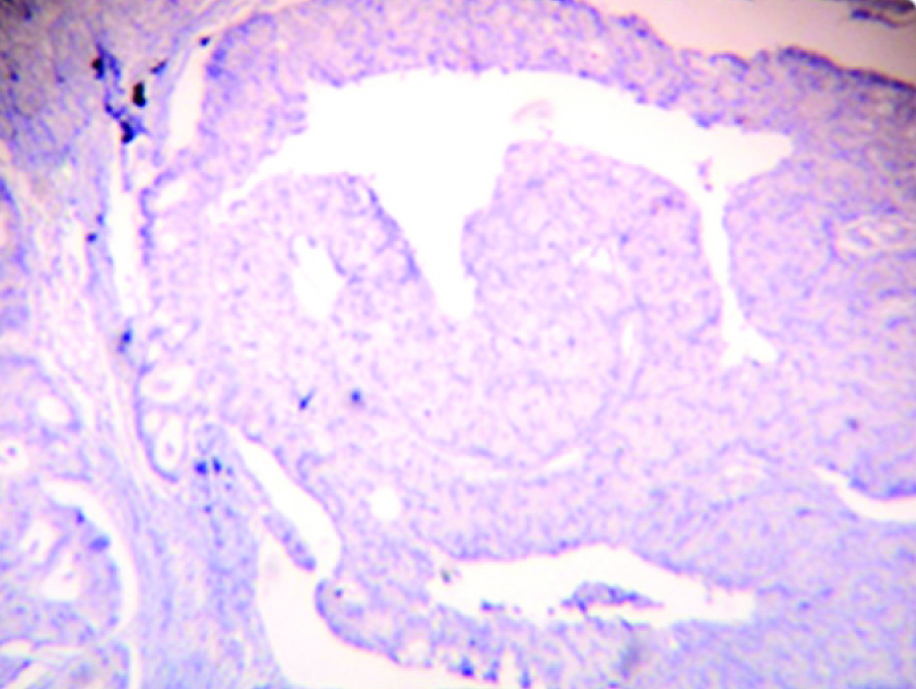
The present study showed significant difference in expression of OPN between the normal oral mucosa & patient matched tumour free surgical margins [Table/Fig-4b]. The tumour free surgical margins showed 55% positive expression in the epithelium, inflammatory cells & stromal cells [Table/Fig-2a–d]. Similar findings were described by Brown et al., & Masloub et al., who stated that the macrophage adjacent to tumour cells were likely the major source of OPN synthesis in the tumour environment [21,22]. Therefore immune staining of tumour cells and stromal cells for OPN protein were prominent at the invasive edge of the tumours.
Apart from the above mentioned findings, various significant observations were made. In the histological section of patient matched tumour free surgical margins, 3 out of 11 cases of lymph node showed positivity for OPN in the germinal centers [Table/Fig-2e-g]. Ito et al., reported an over-expression of OPN in lymph nodes by IHC and found it to be associated with a poor prognosis [23]. In the histological section of patient matched tumour free surgical margins 4 out of 11 cases of muscle tissues showed the OPN expression [Table/Fig-2h-j]. Uaesoontrachoon et al., reported that myoblast cultures with fibroblast growth factor-2, transforming growth factor-β1, interleukin 1-beta or thrombin showed significantly increased OPN expression [24]. These results suggested that myoblasts are an important source of OPN in damaged muscle. In salivary ductal cells of tumour free margin 3 out of 11 cases showed positivity for OPN [Table/Fig-2k,l]. Coppola et al., reported that in salivary gland carcinoma, the expression of OPN was minimal [25].
In our study, one patient who showed positive expression of OPN in tumour free surgical margins had recurrence after one year of follow up. Denhardt et al., stated that OPN produced by both tumour cells and stromal cells could enhance the metastatic capacity of tumour [26]. A role of OPN in facilitating the survival of the metastatic cell is suggested by the ability of OPN to suppress Nitrous oxide (NO) production. Nitrogen metabolites along with reactive oxygen species kill the circulating tumour cells by the phenomenon of oxidative burst of the macrophages. Since OPN causes the suppression of NO this can be directly correlated to the increased survival of the metastatic tumour cells. Rudland et al., suggested the presence of the metastasis associated protein OPN was tightly correlated with patient demise [20]. This could be applied to our case as well.
Various studies have shown that OPN supports attachment for a variety of cell types and promotes migration of tumours cells which has been also reaffirmed by the findings of our present study [27–29].
Limitation
We couldn’t include the tongue cancer specimens as difficult to obtain tumor free surgical margin tissue from posterior margins of the tongue.
Conclusion
This study highlights that OPN can be used as a diagnostic biomarker in the OSCC. OPN expression in tumour free margins can be correlated to local recurrences and a relatively poor prognosis. Further long term studies with survival analyses can help in establishing as a potential prognostic of oral cancer.
[1]. Le QT, Sutphin PD, Raychaudhuri S, Yu SCT, Identification of Osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomasClinical Cancer Research 2003 9:59-67. [Google Scholar]
[2]. Feller L, Lemmer J, Oral squamous cell carcinoma: Epidemiology, clinical presentation and treatmentJ cancer therapy 2012 3:263-68. [Google Scholar]
[3]. Warnakulasuriya S, Prognostic and predictive markers for oral squamous cell carcinoma: The importance of clinical, pathological and molecular MarkersS J Medicine & Medical Sciences 2014 2(1):12-16. [Google Scholar]
[4]. Scully C, Began J, Oral squamous cell carcinoma overviewJ Oral Oncology 2009 45:301-08. [Google Scholar]
[5]. Bree RD, Putten LVD, Brouwer J, Castelijns JA, Detection of locoregional recurrent head and neck cancer after (chemo) radiotherapy using modern imagingJ Oral Oncology 2009 45:301-08. [Google Scholar]
[6]. Blide A, Buchwald CV, Dabelsteen E, Therkildsenn MH, Molecular markers in the surgical margin of oral carcinomasJ Oral Pathol Med 2009 38:72-78. [Google Scholar]
[7]. Senger DR, Wirth DF, Hynes RO, Transformed mammalian cells secrete specific proteins and phosphoproteinsCell 1979 16:885-93. [Google Scholar]
[8]. Bao LH, Sakaguchi H, Fujimoto J, Tamaya T, Osteopontin in metastatic lesions as a prognostic markers in ovarian cancerJ Biomed Sci 2007 14(3):373-81. [Google Scholar]
[9]. Han WH, Wei WX, Can ET, Osteopontin expression in nasopharyngeal carcinoma: Its relevance to the clinical stage of the diseaseJ Cancer Research And Therapeutics 2011 7(2):138-42. [Google Scholar]
[10]. Zhang LL, Shao SL, Wu Y, The expression and significance of Osteopontin and B7-H4 in epithelial ovarian neoplasmChinese Journal of Cancer 2010 29(1):24-28. [Google Scholar]
[11]. Wu IC, Yang SF, Wu CC, Hsu WH, Expression of osteopontin protein in esophageal squamous cell carcinomaJ Intern Med Taiwan 2010 21:419-26. [Google Scholar]
[12]. Conti J, Thomas G, The role of tumour stroma in colorectal cancer invasion and metastasisJ Cancer 2011 3:2160-68. [Google Scholar]
[13]. Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Osteopontin–a molecular for all seasonsQJM: An Int J Med 2002 95(1):3-13. [Google Scholar]
[14]. Packer L, Pavey S, Parker A, Stark M, Osteopontin is a downstream effector of the P13 kinase pathway in melanomas that is inversely correlated with functional PTENCarcinogenesis 2006 27(9):1778-86. [Google Scholar]
[15]. Petrik D, Lavori PW, Cao H, Zhu Y, Plasma Osteopontin Is an Independent prognostic marker for head and neck cancersJournal of Clinical Oncology 2006 24:5291-97. [Google Scholar]
[16]. Chien CY, Su CY, Chuang HC, Fang FM, Clinical significance of osteopontin expression in T1 and T2 Tongue cancersHead & neck 2008 10:776-81. [Google Scholar]
[17]. Ogbureke KUE, Abdelsayed RA, Kushner H, Li Li BA, Fisher LW, Two members of the sibling family of proteins, dspp and bsp, may predict the transition of oral epithelial dysplasia to oral squamous cell carcinomaCancer 2010 116(7):1709-17. [Google Scholar]
[18]. Devoll RE, Wei L, Woods KV, Pinero GP, Osteopontin (OPN) distribution in premalignant and malignant lesions of oral epithelium and expression in cell lines derived from squamous cell carcinoma of the oral cavityJ Oral Patho Med 1999 28:97-101. [Google Scholar]
[19]. Zhou ZT, Wei BJ, Shi P, Osteopontin expression in oral lichen planusJ Oral Pathol Med 2008 37:94-98. [Google Scholar]
[20]. Rudland PS, Higgins AP, Tanani ME, Rudland SDS, Prognostic Significance of the metastasis-associated protein osteopontin in human breast cancerCancer Research 2002 62:3417-27. [Google Scholar]
[21]. Brown LF, Sergiou AP, Berse B, Manseu EJ, Osteopontin expression and distribution in human carcinomasAmerican Journal of Pathology 1994 145(3):610-23. [Google Scholar]
[22]. Masloub SM, Azim AMA, Elhamid ESA, CD10 & Osteopontin expression in dentigerous cyst and ameloblastomaDiagnostic pathology 2011 6:44 [Google Scholar]
[23]. Ito T, Hashimoto Y, Tanka E, Kan T, An inducible short hairpin RNA vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivoClin Cancer Res 2006 12:1308-16. [Google Scholar]
[24]. Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Osteopontin and skeletal muscle myoblast :association with muscle regeneration and regulation of myoblast function in vitroThe international J Biochemistry & Cell Biology 2008 10(40):2303-14. [Google Scholar]
[25]. Coppola D, Szabo M, Boulware D, Muraca P, Correlation of Osteopontin protein expression and pathological stage across a wide variety of tumour HistologiesClinical Cancer Research 2002 10:184-90. [Google Scholar]
[26]. Denhardt DT, Noda M, Regan AWO, Pavlin D, Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling and cell survivalThe Journal of Clinical Investigation 2001 107(9):1055-61. [Google Scholar]
[27]. Kim JH, Skates SJ, Uede T, Wong KK, Osteopontin as a potential diagnostic biomarker for ovarian cancerJAMA 2002 287:1671-79. [Google Scholar]
[28]. Chakraborty G, Jain S, Behera R, Ahmed M, The multifaceted roles of osteopontin in cell signaling, tumour progression and angiogenesisCurr Mol Med 2006 6:819-30. [Google Scholar]
[29]. Rangaswami H, Bulbule A, Kundu GC, Osteopontin: Role in cell signaling and cancer progressionTrends Cell Biol 2006 16:79-87. [Google Scholar]