Background
Enterococcus faecalis is one of the most commonly occurring organisms retrieved from root canal treated teeth that show refractory apical periodontitis. Though it is well known that the ability of E. faecalis to form a matrix-encased biofilm contributes to its pathogenicity, the role of extracellular dextran and DNA in biofilm formation and its effect on the susceptibility of the biofilm to chlorhexidine remains poorly understood. It was hypothesized that the addition of an Extracellular Polymeric Substance (EPS) degrading enzyme along with a detergent to chlorhexidine may increase the susceptibility of the E. faecalis biofilm.
Aim
To evaluate the sensitivity of Enterococcus faecalis biofilms treated with DNase enzyme and their susceptibility to 2% chlorhexidine used alone or in conjunction with a detergent in a dentin disinfection model and examine under confocal laser scanning microscopy (CLSM).
Materials and Methods
Semi cylindrical shaped dentin specimens were infected with E. faecalis and incubated for 24 hours. Following incubation, the infected dentin specimens were exposed for 3 minutes to the four disinfecting solutions and grouped accordingly. {Group I- Sterile saline, Group II- 2% Chlorhexidine (CHX), Group III– Dnase1 Enzyme + 2% CHX, Group IV- DNase1 Enzyme + 2% CHX & Tween 80. Bacterial viability was then assessed by staining the specimens and examining under CLSM to analyse the proportion of dead and live bacteria within the dentinal tubules.
Results
The Groups II, III and IV showed statistically significant (p<0.05) percentage of dead bacteria compared to the control (Group I). However there was no significant difference in the killing effectiveness within the experimental groups (II-IV) at (p<0.05).
Conclusion
EPS degrading enzyme (DNase I) disrupts the biofilm and increases the susceptibility of E.faecalis when exposed to 2% Chlorhexidine and the use of a surfactant with this combination significantly contributes to improving the antibacterial efficacy.
Introduction
Enterococcus faecalis is an anaerobic gram-positive bacteria, has ability to survive under harsh conditions and can penetrate into the dentinal tubules [1,2]. Pinheiro et al., used RT-qPCR assay and identified 77.8% of E. faecalis from teeth with persistent/secondary intraradicular infections [3]. Zhang et al., proved that E. faecalis was highly related to persistent intraradicular infections compared with untreated chronic periapical periodontitis [4].
Biofilms represent bacterial colonies enveloped within an extracellular matrix composed of polysaccharides, proteins, and nucleic acids. The bacteria growing in this environment have advantages such as: i) cell – cell communication which helps in survival and virulence; ii) genetic exchange between cells, which include genes encoding virulence and bacterial resistance against antibiotics; and iii) protection from environmental stress and host defence [5]. Thus E. faecalis biofilms pose a severe challenge to eradication during root canal treatment procedures.
Thomas et al., provided primary evidence for the pivotal role of extracellular DNA (eDNA) in E. faecalis biofilms. eDNA is a quintessential component of the extracellular polymeric substance of bacterial biofilms, providing structural stability and resistance against antimicrobial agents [6]. Li et al., had evaluated the effect of dextran-degrading enzyme DNase I on E. faecalis biofilm and found that it inhibited biofilm formation, decreased its adhesion to dentin and increased its susceptibility to 2% Chlorhexidine [7]. The EPS degrading enzyme could possess the ability to act as biofilm degrading agent, which in turn could improve the ability of the antibacterial agent to act effectively on the E. faecalis biofilm [7].
CHX is a broad-spectrum antimicrobial agent and effective against both gram-positive and gram-negative organisms [8,9]. It possesses the property of substantivity [10]. Substantivity is defined as the prolonged adherence of CHX to oral surfaces, in this case the dentinal tubules and root canal walls, and its slow release at effective doses that guarantees the persistence of its antimicrobial activity. Recently Ma et al., proved that chlorhexidine might be more effective in improving the antibacterial activities of alkaline root canal medicaments against E. faecalis when compared to NaOCl [11]. The essential function of any irrigating solution in endodontic therapy is to eliminate the infecting organism from the root canal system [12]. The addition of surface active agents to the disinfectants helps to reduce the surface tension and increase the wettability of the solutions, thereby increasing their efficacy [13-16]. Irrigants should contact with the dentin walls by physicochemical property called wettability [17]. Wang et al., proved that adding detergent to disinfecting agents would increase its antibacterial efficacy [18].
A new non-invasive dentin disinfection model was proposed by Ma et al., in 2011 in an in-vitro study to establish bacterial presence within the dentinal tubules [19]. In comparison to bacterial invasion models based on culturing techniques that cannot be replicated in the clinical setup [20], this new model provided penetration of bacteria deep into the dentinal tubules by centrifugation and has been implemented in our study to standardize the methodology. The power of centrifugation forces allows for a strong bacterial invasion into the dentin and allows for standardized measurements of live/dead bacteria when used with the CLSM to measure the efficacy of the disinfecting solutions. Confocal Laser Scanning Microscopy (CLSM) presents two distinct advantages over any other microscopic system in its ability to control the depth of field with elimination or reduction of background information from the focal plane thereby avoiding image degradation and its potential to obtain serial optical sections from thick specimens [21]. CLSM helps in visualizing the live and dead bacteria, and has the ability to penetrate 10 μm below the surface of the specimen. Del Carpio-Perochena A et al., used CLSM to measure the cell viability and bacterial volume on polymicrobial biofilms [22].
Hence the aim of this study was to evaluate the susceptibility of E.faecalis biofilms to 2% chlorhexidine that was treated with DNase enzyme, and used alone or in conjunction with a detergent in a dentin disinfection model and examine under Confocal Laser Scanning Microscopy (CLSM).
Materials and Methods
Specimen Preparation
This study was carried out jointly at the Department of Conservative dentistry and Endodontics and the Central research facility of Sri Ramachandra University in the year 2012 after obtaining ethical clearance from the institutional review board. Eight extracted caries free single rooted teeth were used in this study. The teeth were stored in 0.01% sodium hypochlorite solution before use in order to clear them off the soft tissue debris. Based on a previously mentioned protocol [19], a root dentin block, with a length of 4 mm was horizontally sectioned from each tooth at 1 mm below the cementoenamel junction by a 0.6-mm-thick precision diamond saw (Isomet 5000; Buehler Ltd, LakeBluff, IL) at 1000 rpm under water cooling. Each cylindrical dentin block was fractured by first making a thin groove in the middle of the specimen by using low speed handpiece with a small round bur (Tulsa Dentsply) and then fracturing the specimen with a blade and a hammer into 2 semi cylindrical halves. Root canals were enlarged to 1.5 mm with a Gates Glidden drill (Mani Inc, Japan) at 300 rpm under water cooling. Sixteen semi cylindrical halves were thus vertically fractured from the 8 dentin blocks, followed by final shaping and refining of the specimens to obtain a specimen size measuring 4x4x2 mm. The outer surfaces of the 16 semi cylindrical halves were ground by 600-grit silicon carbide paper (Carbine, Buehler Ltd) to achieve a standard thickness of 2 mm and to remove the root surface cementum. The dentin specimens were then shaped by a water-cooled low-speed handpiece with a fine carbide bur (Tulsa Dentsply) at 300 rpm to make the specimen fit the inner wall of a filter tube with 0.45-mm pore size (Pall Corporation, Ann Arbor, MI).
The smear layer on both sides of the specimen was removed by immersion in 5.25% NaOCl (Prime Dental Products, Mumbai, India) and 17% EDTA (Prime Dental Products, Mumbai, India) individually for 4 minutes. The specimens were then rinsed in sterile water for 1 minute. The dentin specimens, following preparation, were placed canal sides up at the bottom of the upper chamber of a microfiltration tube (Pall Corporation, Ann Arbor, MI) and the space between the dentin block and inner tube was sealed by a composite filling material (Synergy D6, Coltene Whaledent) and light cured for 20 seconds [Table/Fig-1].
Specimen preparation and disinfection
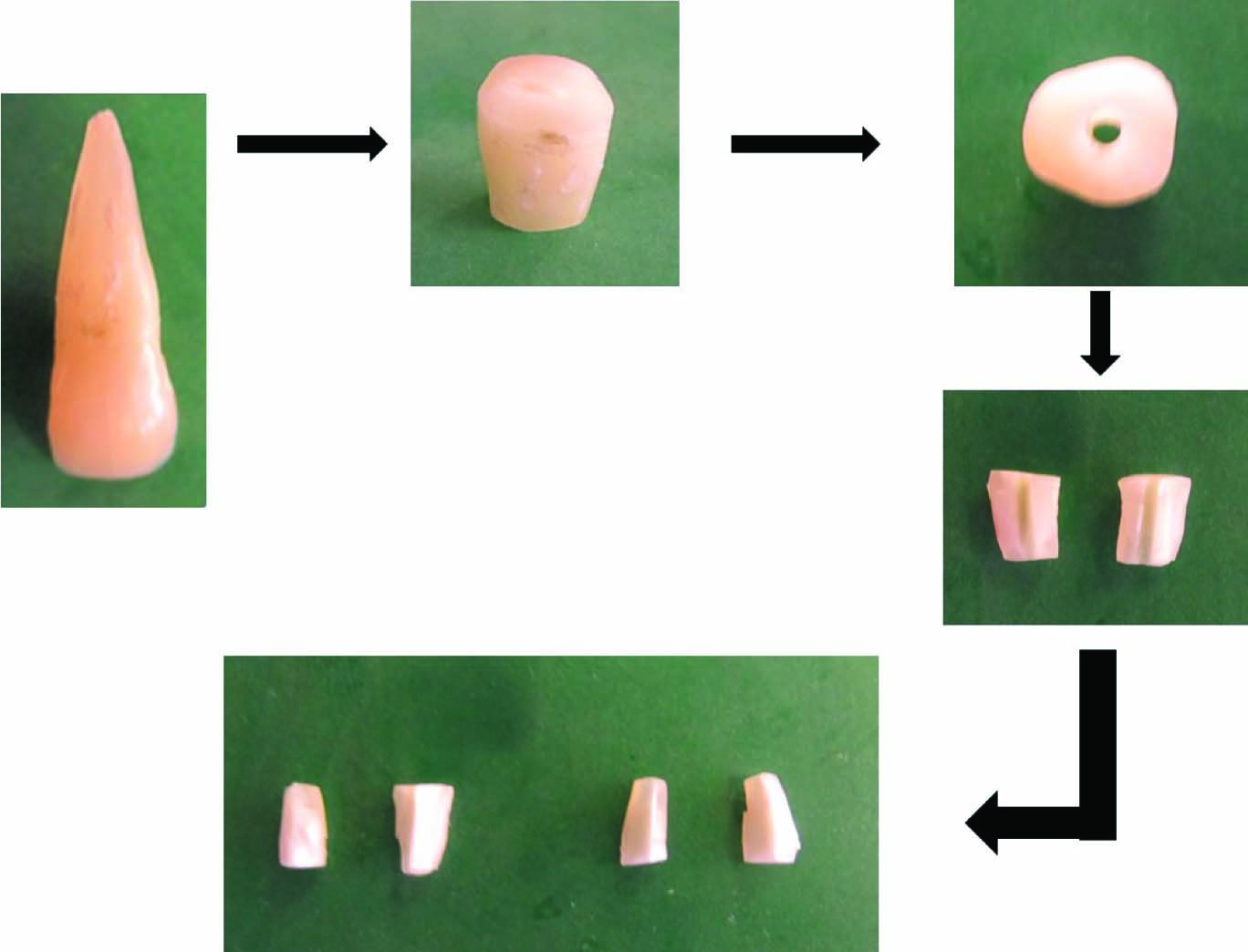
Dentin Infection
E. faecalis (American Type Culture Collection 29212), used as the test organism was grown at 37°C on brain-heart infusion (BHI) agar plates overnight. The organism was standardized spectrophotometrically to McFarlands scale of 0.5 to form 3x106 colony-forming units/mL. The E.faecalis suspension was further sequentially centrifuged into the dentinal tubules. The infected dentin specimens within the tubes were incubated at 37°C in brain-heart infusion broth in air for 24 hours to allow bacterial recovery following centrifugation [19].
Dentin Disinfection
The dentin specimens were removed from the tubes and rinsed in phosphate buffered saline (PBS) for 1minute to remove the broth followed by air-drying. The outer cemental surfaces were coated (2 coats) with clear nail varnish (Lakme). The 16 dentin blocks were randomly assigned into 4groups with 4 specimens in each: Group I- Sterile Saline (Control), GroupII-2% Chlorhexidine (CHX) (ASEP RC, Anabond- Stedman, India), Group III-DNase1 enzyme (Sigma, Activity of 2,200 Kunizunits/mg) + 2% CHX, Group IV- DNase1 Enzyme + {(2% CHX with Tween 80 (“polyoxyethylenesorbitanmonooleate”) in a ratio of 1:1}. Tween 80 is a detergent and is a nonionic surfactant that helps in reducing the surface tension. A 50μL of each of the disinfecting solutions were placed on the root canal side of each dentin specimens for 3 minutes. In groups III & IV, the dentin specimens were incubated for 10 minutes in 1mL BHI broth containing 100 μg/mL of DNase1, following which the disinfecting solutions were applied. The specimens were then rinsed thoroughly in PBS buffer for 1 minute and fractured vertically through the root canal into 2 halves there by exposing a fresh longitudinally fractured dentin wall for CLSM examination [19].
CLSM Examination
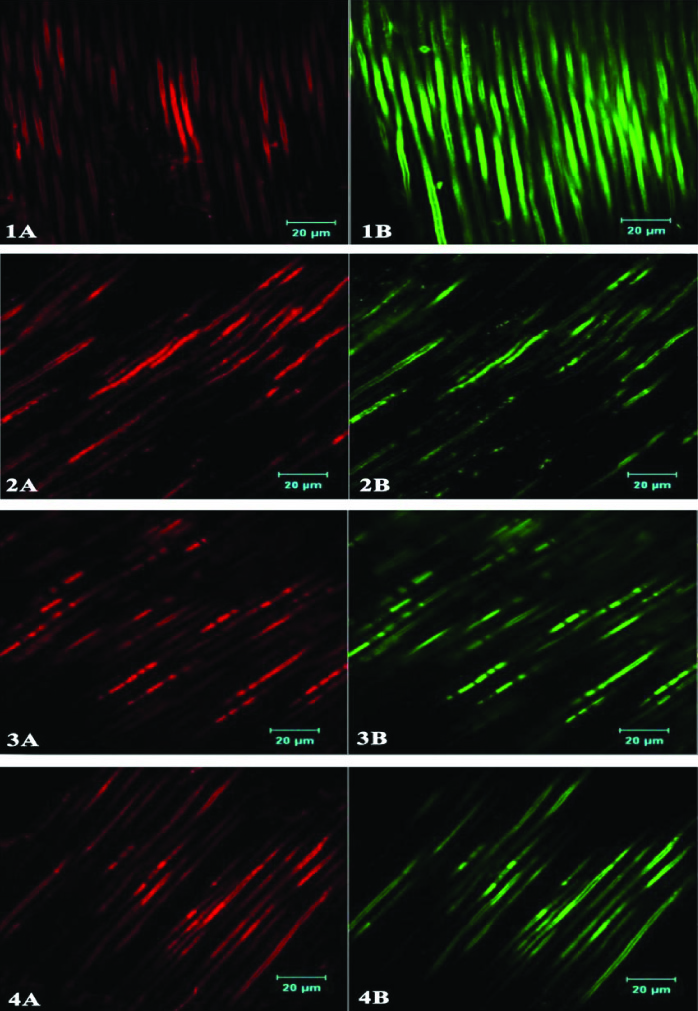
The Confocal microscopy was performed using a LSM710 Metalaser scanning confocal microscope (Carl Zeiss) having a 63x oil immersion objective. The images were acquired and analysed using the AIM software (Carl Zeiss 4.2 version SP.1). For viewing under the confocal microscope, the washed dentin specimens were stained in the dark at room temperature with Fluorescein Diacetate (Sigma) (green fluorescence) and Propidium iodide (Sigma) (red fluorescence) to differentiate the live and dead cells in the bacterial biofilm. The specimens were washed twice with PBS buffer to remove the excess dye. The specimens were then transferred to glass cover slips, and covered with immersion oil before image acquisition. Confocal illumination was performed using an argon laser with excitation wavelength of 543nm for red and 488 nm for green fluorescence. Simultaneous dual-channel imaging mode was used to display both green and red fluorescence. For each group 8 dentin blocks were used for image analysis and each sample was scanned in 5 areas. The AIM software was used to acquire and analyse the images by removing background fluorescence and quantifying the live/dead ratios of the cells within the infected dentinal tubules. The volume of (red to green) fluorescence to red fluorescence indicated the proportion of killed cells for each disinfecting solution [Table/Fig-2].
Confocal armamentarium and bacterial stains used in the study

Statistical Analysis
Statistical analysis was performed using SPSS software (SPSS for Windows version 16, SPSS Inc, Chicago, IL). The acquired images from CLSM were stacked and analysed using one-way ANOVA and multiple comparisons using Turkey HSD test. A p<0.05 was considered to be statistical significant.
Results
An even penetration of E.faecalis extending deep into the dentinal tubules of the specimens was observed by CLSM. The control group, (Group1), treated with sterile saline, showed maximum presence of live bacteria within the dentinal tubules. The Groups II, III and IV showed statistically significant (p<0.05) percentage of killing effectiveness compared to the control (GroupI), with no significant difference in the killing effectiveness within the experimental groups (II-IV) although group III showed higher percentage of dead bacteria compared to groups II & IV after an exposure time of 3 minutes [Table/Fig-3,4].
Figures from 1-4 represent the 4 groups- Group I: Sterile Saline, Group II: 2% CHX, Group III: 2% CHX+DNase I, Group IV: 2% CHX+Tween 80+DNase I. A represents dead bacteria and B represents live bacteria
Percentage of killing effectiveness of E. faecalis cell volume in a dentin disinfection model treated with disinfectant solution
| Groups | Mean & Standard Deviation(Absolute Fluorescence) | Percentage(Killing Effectiveness) |
|---|
| Live | Dead | Live | Dead |
|---|
| Sterile Saline | 8913.63±1930.890 | 676.38±197.414 | 92.71% | 6.07% |
| 2% CHX | 3060.50±881.169 | 5385.25±2324.059 | 38.86% | 61.1% |
| 2%CHX + DNase I | 2000.75±849.904 | 6584.88±2091.893 | 24.08% | 75.85% |
| 2%CHX + Tween80+DNase I | 1533.63±702.296 | 3858.63±733.964 | 27.39% | 73.50% |
Discussion
Enterococci are gram-positive cocci and facultative anaerobes that can occur either in glyorin pairs, or even as short chains [23]. E.faecalis has been associated with different forms of periradicular disease which ranges from primary endodontic infections to persistent periradicular infections [24]. E. faecalis possess a number of virulence factors like lytic enzymes, pheromones, aggregation substance, cytolysin, and lipoteichoic acid [25]. These enable them to survive very challenging environments by resisting all the antimicrobial measures that are used such as irrigants and intracanal medicaments. In addition E.faecalis possess the ability to suppress the action of lymphocytes, thereby contributing to endodontic failure [26]. The presence of serineprotease, gelatinase, and collagen-binding protein (Ace) provides the ability to bind and adhere to dentin [27]. However, the most important factor of E.faecalis is its ability to form a biofilm that prevents it from degradation by enabling the bacterial colony to become 1000 times more resistant to antibodies, phagocytosis, and antimicrobial agents compared to planktonic bacteria [1,28].
The extracellular matrix of biofilms is composed of proteins, carbohydrates and nucleic acids [5,29]. It has been thought that the presence of extracellular polymeric substances (EPS’s) in the bacterial biofilm imparts the bacterial colony resistance against immune system and antibacterial agents [30,31]. The EPS matrix provides the biofilm with mechanical stability and a cohesive, interconnected 3D polymer network. Also, the biofilm matrix serves as an external digestive system by trapping the extracellular enzymes close to the cells which enables them to metabolize dissolved, colloidal and solid biopolymers [31]. In recent years, dextran and eDNA have been identified in the matrix of E.faecalis biofilms suggesting their role in the development of bacterial communities. Dextran belongs to a class of extracellularly formed glucans that is produced by bacteria while eDNA in biofilms is presumably derived from cell lysis [6,32].
From the results of our study it was seen that the dentin biofilms irrigated with 2% chlorhexidine have shown the least percentage of killing effectiveness. In the field of endodontics, CHX has been used as an irrigant with proven substantivity because of its ability to adhere to hydroxyapatite of dentin [33,34] and its effectiveness against endodontic pathogens. However, biofilms within root canals are resistant to the antibacterial effect of CHX. Hence in this study chlorhexidine when used alone has performed poorly. The reason for the superior disinfection of Group III and IV could be attributed to the ability of the DNase1 enzyme to disrupt the bacterial biofilm making it susceptible to the antibacterial effect of 2% chlorhexidine. Thus in the presence of DNase1, it may have been possible for CHX to act more directly on the bacterial biofilm, thereby increasing its bactericidal potential.
Wettability of the disinfecting solutions used during irrigation has also been shown to play a major role in facilitating their penetration into the intricacies of the root canal system [35]. It has been defined as the force between molecules that tends to reduce the surface area of a liquid [36]. This decrease in the surface tension which is achieved by the addition of chemical compounds known as detergents [37] which can disintegrate the cohesive forces and promote the destruction of EPS matrix and bacterial cell membrane [38]. Detergents such as Cetrimide and Tween 80 have been employed in endodontic irrigants such as CHX-Plus and MTAD respectively to decrease their surface tension [39]. Previous studies have revealed that addition of detergents with inherent antibacterial activity to the disinfecting solutions have significantly increased their antibacterial effect on dentin biofilms [18]. The results of our study have also shown a marginal increase in the antibacterial efficacy in the surfactant added group (Group IV) compared with Group III (2% chlorhexidine + DNase1 enzyme) though not statistically significant. The surfactant (Tween 80) used in the present study did not possess any inherent antibacterial properties but still has shown improved antibacterial activity when added to 2% chlorhexidine. The addition of DNase1 enzyme which disrupts the bacterial biofilm has made it susceptible to the antibacterial effect of 2% chlorhexidine. In addition the use of a surfactant along with the DNase I enzyme has enabled deeper penetration of 2% chlorhexidine into the dentinal tubules by lowering the surface tension and increasing the wettability of the chlorhexidine. Hence the superior performance of the experimental groups can be explained. The results of our study have thus shown a definite increase in antibacterial activity with the use of both DNase1enzyme and the surfactant along with 2% Chlorhexidine.
Conclusion
Within the limitations of our study, that include the use of only one single strain of E. faecalis biofilm and the inherent difficulty of extrapolating the results obtained by an in-vitro investigation, to an invivo clinical setup, it can be concluded that, the use of an EPS degrading enzyme (DNase I) disrupts the biofilm and increases the susceptibility of E. faecalis when exposed to 2% chlorhexidine and the use of a surfactant with this combination significantly contributes to improving the antibacterial efficacy.
[1]. Stuart CH, Schwartz SA, Beeson TJ, Owatz CB, Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatmentJ Endod 2006 32(2):93-98. [Google Scholar]
[2]. George S, Kishen A, Song KP, The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalisJ Endod 2005 31(12):867-72. [Google Scholar]
[3]. Pinheiro ET, Candeiro GT, Teixeira SR, Shin RC, Prado LC, Gavini G, RNA-based assay demonstrated enterococcus faecalis metabolic activity after chemomechanical proceduresJ Endod 2015 Jun 9 doi: 10.1016/j.joen.2015.04.020 [Google Scholar]
[4]. Zhang C, Du J, Peng Z, Correlation between Enterococcus faecalis and persistent intraradicular infection compared with primary intraradicular infection: A Systematic ReviewJ Endod 2015 41(8):1207-13. [Google Scholar]
[5]. Siqueira JF, Rôças IN, Ricucci D, Biofilms in endodontic infectionEndod Top 2012 22:33-49. [Google Scholar]
[6]. Thomas VC, Thurlow LR, Boyle D, Hancock LE, Regulation of autolysis- dependant extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm developmentJ Bacteriol 2008 190(16):5690-98. [Google Scholar]
[7]. Li W, Liu H, Xu Q, Extracellular dextran and DNA affect the formation of Enterococcus faecalis biofilms and their susceptibility to 2% ChlorhexidineJ Endod 2012 38(7):894-98. [Google Scholar]
[8]. Karpinski TM, Szkaradkiewicz AK, Chlorhexidine–pharmaco-biological activity and applicationEur Rev Med Pharmacol Sci 2015 19(7):1321-26. [Google Scholar]
[9]. Carbajal Mejía JB, Antimicrobial effects of calcium hydroxide, chlorhexidine, and propolis on Enterococcus faecalis and Candida albicansJ Investig Clin Dent 2014 5(3):194-200. [Google Scholar]
[10]. Mahendra A, Koul M, Upadhyay V, Dwivedi R, Comparative evaluation of antimicrobial substantivity of different concentrations of Chlorhexidine as a root canal irrigant: An in vitro studyJ Oral Biol Craniofac Res 2014 4(3):181-85. [Google Scholar]
[11]. Ma J, Tong Z, Ling J, Liu H, Wei X, The effects of sodium hypochlorite and chlorhexidine irrigants on the antibacterial activities of alkaline media against Enterococcus faecalisArch Oral Biol 2015 60(7):1075-81. [Google Scholar]
[12]. Haapasalo M, Endal U, Zandi H, Coil J, Eradication of endodontic infection by instrumentation and irrigation solutionsEndod Topics 2005 10:77-102. [Google Scholar]
[13]. Lui JN, Kuah HG, Chen NN, Effect of EDTA with and without surfactants or ultrasonics on removal of smear layerJ Endod 2007 33(4):472-75. [Google Scholar]
[14]. Palazzi F, Morra M, Mohammadi Z, Grandini S, Giardino L, Comparison of the surface tension of 5.25% sodium hypochlorite solution with three new sodium hypochlorite based endodontic irrigantsInt Endod J 2011 45(2):129-35. [Google Scholar]
[15]. Bukiet F, Couderc G, Camps J, Tassery H, Cuisinier F, About I, Charrier A, Candoni N, Wetting properties and critical micellar concentration of benzalkonium chloride mixed in sodium hypochloriteJ Endod 2012 38(11):1525-29. [Google Scholar]
[16]. Stojicic S, Zivkovic S, Qian W, Zhang H, Haapasalo M, Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactantJ Endod 2010 36(9):1558-62. [Google Scholar]
[17]. Hu X, Ling J, Gao Y, Effects of irrigation solutions on dentin wettability and roughnessJ Endod 2010 36(6):1064-67. [Google Scholar]
[18]. Wang Z, Shen Y, Ma J, Haapasalo M, The effect of detergents on the antibacterial activity of disinfecting solutions in dentinJ Endod 2012 38(7):948-53. [Google Scholar]
[19]. Ma J, Wang Z, Shen Y, Haapasalo M, A new non invasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopyJ Endod 2011 37(10):1380-85. [Google Scholar]
[20]. Shen Y, Qian W, Chung C, Olsen I, Haapasalo M, Evaluation of the effect of two Chlorhexidine preparations on biofilm bacteria invitro: a three dimensional quantitative analysisJ Endod 2009 35(7):981-85. [Google Scholar]
[21]. Amos WB, White JG, How the Confocal laser scanning microscope entered biological researchBiol Cell 2005 95(6):335-42. [Google Scholar]
[22]. Del Carpio-Perochena A, Bramante CM, de Andrade FB, Maliza AG, Cavenago BC, Marciano MA, Antibacterial and dissolution ability of sodium hypochlorite in different pHs on multi-species biofilmsClin Oral Investig 2015 Feb 26 DOI: 10.1007/s00784-015-1431-36 [Google Scholar]
[23]. Gilmore MS, The Enterococci: pathogenesis, molecular biology, and antibiotic resistance 2002 WashingtonASM Press [Google Scholar]
[24]. Rôças IN, Siqueira JF, Santos KRN, Association of Enterococcus faecalis with different forms of periradicular diseasesJ Endod 2004 30(5):315-20. [Google Scholar]
[25]. Sedgley CM, Lennan SL, Clewell DB, Prevalence, phenotype, and geno type of oral EnterococciOral Microbiol Immunol 2004 19:95-101. [Google Scholar]
[26]. Lee W, Lim S, Son H, Bae K, Sonicated extract of Enterococcus faecalis induces irreversible cell cycle arrest in phyto hemagglutinin - activated human lymphocytesJ Endod 2004 30(4):209-12. [Google Scholar]
[27]. Hubble TS, Hatton JF, Nallapareddy SR, Murray BE, Gillespie MJ, Influence of Enteroccocus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentinOral Microbiol Immunol 2003 18:121-26. [Google Scholar]
[28]. Stewart PS, Costerton JW, Antibiotic resistance of bacteria in biofilmsLancet 2001 358(9276):135-38. [Google Scholar]
[29]. Chambless JD, Hunt SM, Stewart PS, A three-dimensional computer model of four hypothetical mechanisms protecting biofilms from antimicrobialsAppl Environ Microbiol 2006 72(3):2005-13. [Google Scholar]
[30]. Donlan RM, Costerton JW, Biofilms: survival mechanisms of clinically relevant microorganismsClin Microbiol Rev 2002 15:167-93. [Google Scholar]
[31]. Flemming HC, Wingender J, The biofilm matrixNat Rev Microbiol 2010 8(9):623-33. [Google Scholar]
[32]. Thomas VC, Hiromasa Y, Harms N, Thurlow L, Tomich J, Hancock LE, A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalisMol Micro biol 2009 72(4):1022-36. [Google Scholar]
[33]. Tervit C, Paquette L, Torneck CD, Basrani B, Friedman S, Proportion of healed teeth with apical periodontitis medicated with two percent Chlorhexidine gluconate liquid: a case series studyJ Endod 2009 35(9):1182-85. [Google Scholar]
[34]. Lee Y, Han SH, Hong SH, Lee JK, Ji H, Kum KY, Antimicrobial efficacy of a polymeric Chlorhexidine release device using in vitro model of Enterococcus faecalis dentinal tubule infectionJ Endod 2008 34(7):855-58. [Google Scholar]
[35]. Giardino L, Ambu E, Becce C, Rimondini L, Morra M, Surface tension comparison off our common root canal irrigants and two new irrigants containing antibioticJ Endod 2006 32(11):1091-93. [Google Scholar]
[36]. Tasman F, Cehreli ZC, Ogan C, Etikan I, Surface tension of root canal irrigantsJ Endod 2000 26(10):586-87. [Google Scholar]
[37]. Rossi-Fedele G, Prichard JW, Steier L, de Figueiredo JA, The effect of surface tension reduction on the clinical performance of sodium hypochlorite in endodonticsInt Endod J 2013 46(6):492-98. [Google Scholar]
[38]. Arias-Moliz MT, Ferrer-Luque CM, Gonzalez- Rodriguez MP, Valderrama MJ, Baca P, Eradication of Enterococcus faecalis biofilms by cetrimide and ChlorhexidineJ Endod 2010 36(1):87-90. [Google Scholar]
[39]. Pappen FG, Shen Y, Qian W, Leonardo MR, Giardino L, Haapasalo M, Invitro antibacterial action of Tetraclean, MTAD and five experimental irrigation solutionsInt Endod J 2010 43(6):528-35. [Google Scholar]