Cirrhosis is the end-stage consequence of fibrosis of the liver parenchyma that results in nodule formation and altered hepatic function [1]. For the diagnosis of cirrhosis, liver biopsy is currently the gold standard for assessment of hepatic necro inflammatory activity and fibrosis. This is an invasive procedure subject to inter-observer variability and sampling error of biopsies; biopsy length and fragmentation influences its reliability and histopathological results. There are a number of other complications like bleeding, pneumothorax, infective peritonitis which limit the use of liver biopsies in all patients [2]. Several non-invasive biochemical tests like Fibro Test [3], hepascore, transient electrography [4], fibrospect, forns index, AAR, ELF etc. are currently in use [5–7], requiring complex calculations and expensive biochemical assays.
An ideal non-invasive diagnostic test for hepatic fibrosis should be simple, readily available, reproducible, inexpensive, and accurate. Aspartate aminotransferase: Platelet Ratio Index (APRI) was reported as a novel index for prediction of significant fibrosis and cirrhosis [8,9]. APRI as simple bedside diagnostic tool has been successfully evaluated in Western [10,11] and some Asian populations [12]. But not many studies have been done in Indian population. Hence, in the present study we tried to evaluate APRI as a non-invasive bedside marker of cirrhosis in a subset of Indian population and statistically determine its sensitivity and specificity as a diagnostic tool.
Materials and Methods
The study was conducted in the Medicine Department of Vardhman Mahavir Medical College and Safdarjang Hospital, New Delhi. Total 51 patients of liver cirrhosis as cases and 50 normal subjects as controls (>18 years, both sexes) were considered for study. From January 2009 to April 2010, two hundred patients attending the OPD or admitted in medicine wards with signs, symptoms and investigations suggestive of liver pathology were subjected to ultrasound. On the basis of ultrasonographic findings 51 patients were prospectively enrolled for the study (cases). Healthy controls were selected from the relatives of the patients and other volunteers. The exclusion criteria were liver disorders other than cirrhosis, accompanying illnesses like haematological disorders, malignancy, and chronic disorders like diabetes, hypertension, cardiac diseases, renal failure, any surgical history or patient’s unwillingness to participate in the study.
The USG criteria for the diagnosis of cirrhosis were nodular hepatic contour, enlargement of caudate and lateral segment of left lobe, atrophy of right and medial segment of left lobe, prominence of fissures and porta-hepatis, increased parenchymal echogenicity, decreased ultrasound beam penetration, poor depiction of intrahepatic vessels, regenerating nodules, altered gall bladder angle, loss of normal triphasic hepatic vein Doppler tracing, increased pulsations of portal vein on Doppler tracing and patients with presence of any 5 out of them were labelled as cirrhotic [13]. Ultrasonography was done using a real time 3.5 MHz probe in fasting state in all subjects. Liver size, echo texture, echogenicity, portal vein diameter and presence of collaterals etc were evaluated; preferably by the same observer to minimize inter observer variations. The sensitivity and specificity of USG for cirrhosis is 91.1% and 93.1% respectively with 92.3% accurary [14].
A written informed consent was obtained was obtained from all patients. The institutional ethics committee approved the study protocol. Liver function test comprised of parameters like SGOT, SGPT, Alkaline phosphatase, and serum bilirubin, performed using a fully automatic HITACHI-912 Auto Analyser.
Platelet count was determined by the fully automatic SYSMEX KX 21 Auto analyser using the principle of optical impedance and flow cytometry.
APRI Score was calculated as [8]:
{(AST/upper limit of normal)/platelet count (109/L)} × 100.
Statistical Analysis
Statistical analysis was done using SSPS 17(SSPS software Inc., Chicago, USA). Student t - test was used to compare between two groups in case of quantitative data Receiver Operating Characteristic (ROC) curve was used to see the cut point for APRI and PPV (Positive Predictive Value), NPV (Negative Predictive Value) was calculated where the sensitivity and specificity was optimal. p-value < 0.05 was considered significant.
Results
The demographic profile of the cases and controls is given in [Table/Fig-1].
Comparison of variables in case & control cohort
| Parameters | Case cohort | Control cohort | p-value |
|---|
| Age (years) | 43.08 ± 12.3 | 37.74 ± 12.4 | - |
| Sex (M:F) | 41:10 | 37:13 | - |
| Platelet Count (lac/mm3) | 0.97 ± 0.49 | 2.56 ± 0.8 | <0.001 |
| S. Biliribin(mg/dl) | 4 ± 4.51 | 0.78 ± 0.19 | <0.001 |
| SGOT (AST)(IU) | 73.38 ± 36.93 | 33.8 ± 7.48 | <0.001 |
| SGPT (ALP)(IU) | 44.12 ± 17.86 | 36.8 ± 6.6 | 0.008 |
| Prothrombin time (sec.) | 20.23 ± 9.65 | 13.28 ± 0.86 | <0.001 |
| INR | 1.78 ± 0.98 | 1.02 ± 0.06 | <0.001 |
| APRI Score | 2.178 ± 1.224 | 0.364 ± 0.137 | <0.001 |
| S. Albumin(g/dl) | 2.46 | 3.65 | <0.001 |
All values are Mean ± SD where APRI- Aspartate aminotransferase platelet ration index;INR-international Normalised Ratio; AST-Aspartateaminotransferase; ALT-Aspartate alaninetransferase
Out of 51 patients 9 (17.64%) were Hepatitis B positive, 7 (13.72%) were Hepatitis C positive & 25 (49.01%) were chronic alcoholics, rest of them were not fitting in any particular diagnosis.
There were significant differences in the platelet count, SGOT/PT, INR, Prothrombin time, Albumin and serum bilirubin of the cases and controls (p<0.05) as given in [Table/Fig-1].
The mean APRI score of the case cohort and control cohort were 2.178±1.224 and 0.364±0.137 respectively. The difference was statistically significant (p-value = <0.001, [Table/Fig-1]).
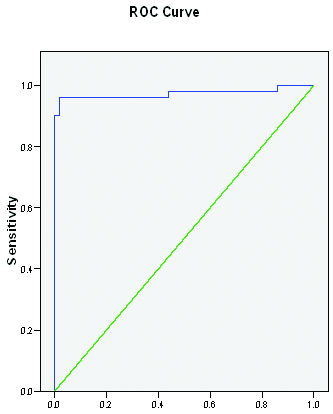
ROC curve for diagnostic accuracy of the APRI model in predicting cirrhosis is given in [Table/Fig-2,3]. The diagnostic accuracy of the APRI model in predicting cirrhosis was found to be 96.1% sensitive and 96% specific [Table/Fig-4].
ROC curve for diagnostic accuracy of the APRI model in predicting cirrhosis
| APRI | Area (95% CI) | p-value |
|---|
| ROC curve | 0.973 (0.936-1.010) | <0.001 |
APRI- Aspartate aminotransferase-to-platelet ratio index, ROC- Receiver Operating Characteristic
Receiver Operating Characteristic (ROC) Curve for Aspartate aminotransferase-to-platelet ratio index (APRI) as a non-invasive diagnostic tool for cirrhosis
Diagnostic accuracy of the APRI model in predicting cirrhosis
| For prediction of cirrhosis | TEST | CONTROL | SENSITIVITY | SPECIFICITY | PPV | NPV |
|---|
| < 0.65 | 2 | 48 | 96.1 l% | 96% | 96.10% | 96% |
| >0.65 | 49 | 2 | | | | |
APRI- Aspartate aminotransferase-to-platelet ratio index; PPV-positive predictive value; NPV-negative predictive value.
Discussion
The present study evaluated the accuracy of APRI as a bedside non-invasive marker for cirrhosis in Indians and our study showed that APRI is a fair and accurate non-invasive marker for cirrhosis with high specificity and sensitivity. The low cost and easy availability of its two variables (AST and platelets) makes APRI a useful and simple bedside test [15].
Based on the ROC [Table/Fig-2,3], a cut-off point was chosen to predict the absence (APRI<0.65) or presence (APRI>0.65) of cirrhosis. For patients with APRI of 0.65 or less, 48 out of 50 (96%) patients will not have cirrhosis in the control cohort and only 2 out of 51 (3.9%) patients in the case cohort with cirrhosis were classified false negative. On the other hand, for patients with APRI > 0.65, 49 out of 51 (96.1%) patients in the case cohort had cirrhosis, and only 2 out of 50 (4%) subjects in the control cohort without cirrhosis were identified as false negative [Table/Fig-4]. The Area under the curve (AUC) of APRI for predicting cirrhosis was 0.973. In our study sensitivity and specificity of APRI was found to be 96.1% and 96% respectively. Also the positive and negative predictive values were 96.1% and 96%respectively. Whereas Wai et al., have found that using the cut-off APRI values of 1.00 and 2.00, as determined by the ROC curves, significant fibrosis could be predicted accurately in 51% and cirrhosis in 81% of patients. The AUC of APRI for predicting significant fibrosis and cirrhosis in the validation set were 0.88 and 0.94, respectively [8]. In a study by Khan et al., cut-offs of APRI score were 0.90 and 1.75 based on ROC for advanced fibrosis. An APRI value ≤0.90 to rule out advanced fibrosis was 90% sensitive and 70% specific with NPV 95% and PPV 49% [9].
Recently, Martin and co-workers have concluded that a combination and non-invasive serum and imaging markers have sufficient accuracy to identify patients with cirrhosis [11]. APRI is also closely associated with liver cirrhosis in patient’s undergoing surgery for hepatocellular carcinoma [16].
In a study by Verma et al., they found significant correlation between APRI and hepatic venous pressure gradient (HVPG) with the median APRI score of 1.19 (range 0.17–7.92). The APRI of ≥1.09 was 66% sensitive and 73% specific with positive and negative predictive value of 85% and 47% respectively [17]. Various studies have compared different type of non-invasive biomarkers available for diagnosis of liver cirrhosis [4,6,17–19]. Sebastini et al., found fibrotest to be more accurate than APRI whereas Lackner et al., showed greater accuracy of APRI over AST: ALT ratio [5,20]. Ucar et al., suggested that APRI has better diagnostic value in patients with significant fibrosis as compared to other serum markers [18]. However, Xia Zhu et al., have concluded that Fibroscan is a reliable and superior predictor of cirrhosis when compared to APRI [19].
Meta analysis by Jin et al., suggested limited value of APRI in identifying Hepatitis B related significant fibrosis and cirrhosis [12]. On the contrary others have shown APRI to be most accurate and simple marker for predicting significant fibrosis in chronic Hepatitis B [6,21]. APRI correlates fairly with HPVG in cirrhotic patients [17]. Radiological and serum markers also correlate well with biopsy scores [22]. APRI can also predict significant fibrosis in patients with Hepatitis C and HIV co-infection [10], whereas, a meta-analysis by Lin et al., suggested moderate degree of accuracy in identifying Hepatitis C related significant fibrosis [23].
Limitations
This study has a few limitations. Firstly, the patients of cirrhosis in the case cohort were taken using the ultrasonography technique instead of liver biopsy, which is the gold standard for the diagnosis of cirrhosis and secondly, the comparison was made with healthy subjects. Ours being tertiary care hospital, patients come to us in very serious and final stages of their illness, hence liver biopsy could not be done. However, the purpose of this study was to avoid the need of biopsy in predicting cirrhosis. Any future study will take up the issue of histopathological diagnosis of cirrhosis where APRI score can be correlated with the grade of fibrosis.
Most of the previous study we’ve come across included the patients of Hepatitis C. We included patients of cirrhosis caused by other diseases which are prevalent in India. A further study which will try to find a relationship of APRI score with patients of cirrhosis caused by different aetiology will be interesting.
Conclusion
A simple index like APRI, consisting of 2 readily available laboratory results (AST level and platelet count), can predict cirrhosis with high degree of accuracy. Besides being non-invasive, it can be determined at the bedside. It can also be of use in areas where facilities for liver biopsy and advanced imaging techniques are not available. However, further prospective studies are needed to validate the APRI in a larger number of patients in other institutes.
All values are Mean ± SD where APRI- Aspartate aminotransferase platelet ration index;INR-international Normalised Ratio; AST-Aspartateaminotransferase; ALT-Aspartate alaninetransferase
APRI- Aspartate aminotransferase-to-platelet ratio index, ROC- Receiver Operating Characteristic
APRI- Aspartate aminotransferase-to-platelet ratio index; PPV-positive predictive value; NPV-negative predictive value.