Liver transplantation is the main therapeutic option for patients with end-stage liver diseases (ESLD) [1]. Pathological evaluation of the liver allograft biopsies plays an integral role in the management of liver allograft recipients [2]. Liver allograft biopsies are performed in response to changes in liver enzyme levels, abnormality in liver function parameters, imaging or functional abnormalities and as follow up or protocol biopsies [3].
Materials and Methods
Retrospective study was carried out from January 2010 to July 2014. A total of 57 needle biopsies were obtained from 35 patients. One biopsy was inadequate hence, it was excluded. All biopsies were performed under ultrasound guidance using 18 gauge liver biopsy needle. Specimen was fixed in 10% buffered formaldehyde and embedded in paraffin-wax. Paraffin sections were cut at 3 μm thicknesses and stained with Haematoxylin and Eosin (H&E), Periodic Acid Schiff (PAS), Gomori’s trichrome (GMT), Prussian blue, orcein and reticulin. Immunohistochemical studies such as cytokeratins CK 7/19 (epithelial markers to show bile ducts), CMV (Cytomegalovirus), HBV (Hepatitis B virus) were carried out if needed. The biopsy was considered adequate if six portal tracts were identified in one section.
Preservation-reperfusion injury (PRI) was characterized by liver-cell ballooning and centrilobular cholestasis. Acute cellular rejection (ACR) was characterized by predominant portal-based lesions, including the classical triad of mixed inflammatory cell infiltrates, venous endothelial inflammation and inflammatory infiltration of bile ducts. Chronic rejection (CR) was characterized by ductopenia and obliterative arteriopathy. HCV recurrence was characterized by portal lymphoid follicle, focal duct damage and mild fatty changes.
Statistical Analysis
Data was collected using IBM SPSS 20. Continuous data was expressed as mean ± SD and range. Non-continuous data was expressed in percentage and numerical values.
Results
Out of 83 liver allograft recipients, 35 recipients were subjected to 57 needle biopsies. One (1.75%) biopsy was inadequate, thus 56 biopsies were considered for study. Out of 35 patients, 22 patients were subjected to single biopsy procedure, 7 patients were subjected to biopsy twice; 3 patients were subjected to biopsy thrice; and 3 patients were subjected to biopsy four times. The distribution of liver biopsies performed in each year is illustrated in [Table/Fig-1].
Depicting frequency of biopsies performed annually
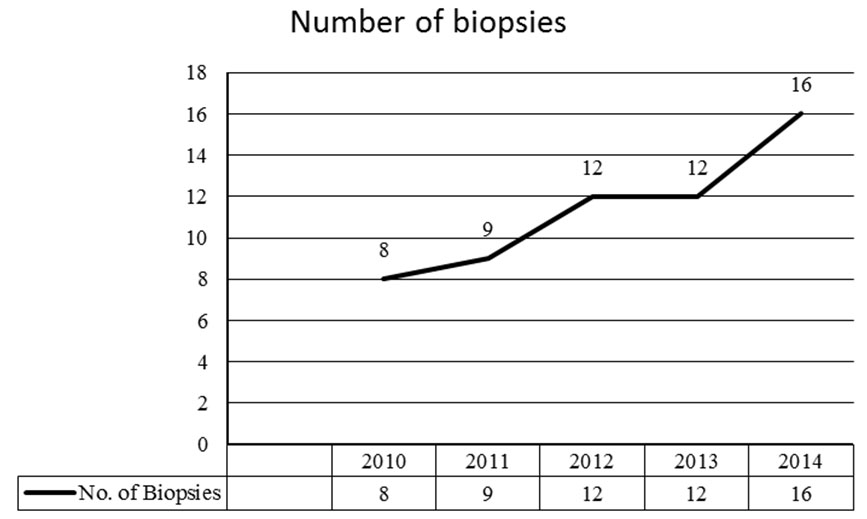
Out of 35 recipients 26 were males and 9 were females. The mean age was 53.2 ± 5.48 years. In laboratory parameters, the mean serum bilirubin was 5.54 ± 7.2 mg/dl, Alanine Transaminase (ALT) was 298 ± 566 IU/L, Aspartate Transaminase (AST), 197 ± 287 IU/L and Alkaline Phosphatase (ALP) was 256 ± 188 IU/L [Table/Fig-2].
Demographics of patients subjected to liver allograft biopsies
| M/F | 26/09 |
| Age (years) | 53.2±5.48 |
| S.Bilirubin (mg/dl) | 5.54 ± 7.2 |
| ALT (IU/L) | 298 ± 566 |
| AST (IU/L) | 197 ± 287 |
| ALP (IU/L) | 256 ± 188 |
The most common indications of liver transplant were alcoholic cirrhosis in 9 (25.71%), cryptogenic cirrhosis in 6 (17.14%), HBV in 6 (17.14%), Wilson disease in 4 (11.42%) and HCV in 3 (8.57%) patients. Decompensated cirrhosis and autoimmune hepatitis were reported in 2 (5.71%) each. Non-alcoholic steatohepatitis (NASH), primary sclerosing cholangitis (PSC) and Primary Hyperoxaluria was noted in 1 (2.85%) each [Table/Fig-3]
Common indications of liver transplant
| Cause of Transplant | n=35 | Percentage % |
|---|
| Alcoholic cirrhosis | 9 | 25.71 |
| Cryptogenic | 6 | 17.14 |
| HBV | 6 | 17.14 |
| WILSON | 4 | 11.42 |
| HCV | 3 | 8.57 |
| Decompensated cirrhosis | 2 | 5.71 |
| Auto immunehepatitis | 2 | 5.71 |
| NASH | 1 | 2.85 |
| PSC | 1 | 2.85 |
| Primary Oxalosis | 1 | 2.85 |
The histological lesions are presented in [Table/Fig-4]. Postoperative time interval for biopsy ranged from first postoperative day to 980 days post-transplant. The most common lesion was ACR [Table/Fig-5] reported in 31 (55.36%) biopsies and intensity ranged from mild ACR in 17 (54.83%), moderate ACR in 12 (38.71 %) and severe ACR in 2 (6.45 %) biopsies [Table/Fig-6]. Twenty three (74%) biopsies with diagnosed ACR were obtained within 180 days post transplant and 8 (26%) were obtained after 180 days post transplant.
| Diagnosis | Biopsy(n=56) | Percentage(%) | Daysmean ± SD | Range(days) |
|---|
| ACR | 31 | 55.36 | 140.94 ±208.79 | 7- 980 |
| PRI | 10 | 17.86 | 24 ± 19.2 | 5-56 |
| Drug toxicity | 8 | 14.29 | 265.12 ± 146.2 | 72-431 |
| HCV recurrence | 3 | 5.36 | 268.7 ± 314 | 26-712 |
| CR | 2 | 3.57 | 247 ±61.5 | 203-290 |
| Acute cholangitis | 1 | 1.79 | 27 | |
| Ischemic Necrosis | 1 | 1.79 | 1 | |
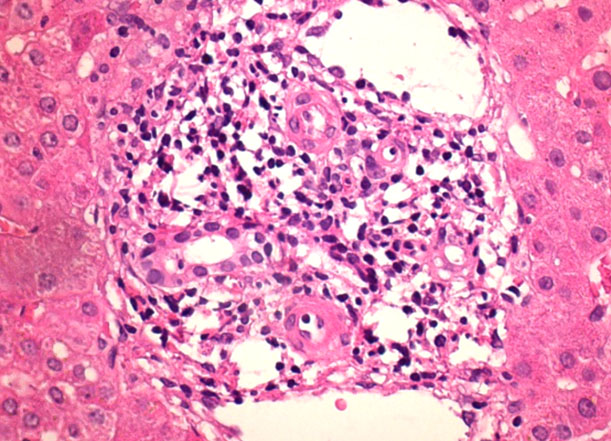
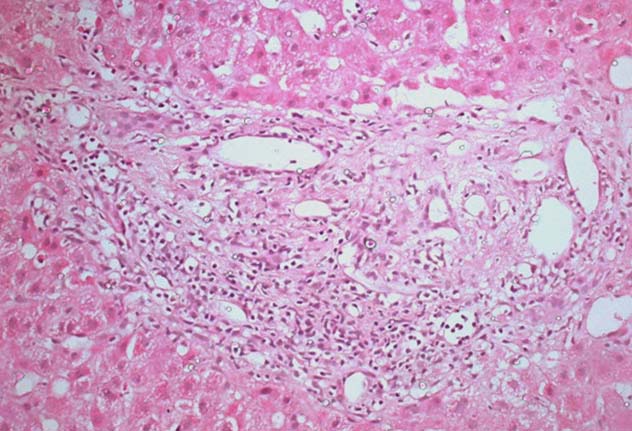
Acute cellular rejection observed 1 month Post-Transplantation, diagnosed by presence of mixed cellular inflammatory infiltrate in portal area along with bile duct infiltration and venous endothelial inflammation, (H&E, X 400)
Duration and types of ACR
| ACR | Number | Percentage (%) | Days | Percentage % |
|---|
| 180 days | 23 | 74 % | | |
| ≥ 180 days | 8 | 26 % | | |
| MILD (RAI 3-4) | 17 | 54.83 % | 175.94 ± 234.88 | 11-980 |
| MODERATE (RAI5-6) | 12 | 38.71 % | 89.75 ± 159.11 | 7-529 |
| SEVERE (RAI ≥ 7) | 2 | 6.45 % | 150.5 ± 197.28 | 11-290 |
PRI or functional cholestasis was observed in 10 (17.86%) biopsies [Table/Fig-7] and drug toxicity [Table/Fig-8] in 8 (14.29%) biopsies. CR [Table/Fig-9] was observed in 2 (3.57%) biopsies performed on day 203 and day 290 posttransplant. HCV recurrence was reported in 3 (5.36%) biopsies. Ischemic coagulative necrosis was observed in one (1.79 %) biopsy on first post-transplant day. Acute cholangitis was also seen in one (1.79 %) biopsy 27 days posttransplant.
Preservation reperfusion injury observed in 5 Days old liver allograft, depicted by liver-cell ballooning and centrilobular cholestasis, (H&E, X 400)
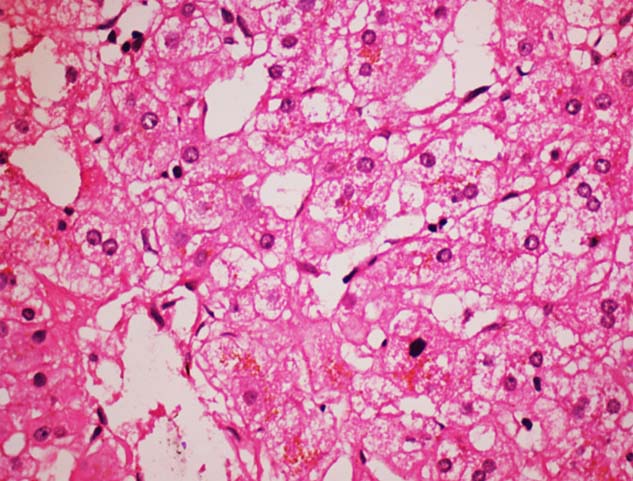
Drug toxicity depicted by microvesicular steatosis in a biopsy performed at 1 year post- transplantation (H&E, X 200)
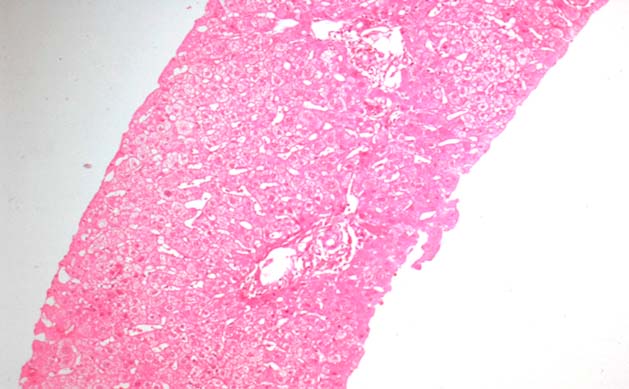
Chronic rejection at 290 days post-transplant, characterized by marked bile duct epithelial damage and ductopenia (depicted by circle) (H&E, X 200)
Discussion
Liver biopsy is the gold standard for diagnosis of allograft dysfunction [4]. Male patients outnumbered females in our study. Indication of the liver biopsies in our study was mainly elevated liver enzymes and/or serum bilirubin. Alcoholic cirrhosis was the most common cause for liver transplant in the present study. However, others have reported HCV as the major cause of ESLD requiring transplantation [5,6]. The histological pattern of acute rejection was first described by Snover et al., [7]. ACR was graded according to Banff schaema for grading liver allograft rejection-1997 [6]. Criteria for evaluating CR was based on Banff Schaema published in 2000 [8].
ACR (55.36%) was the most common histological lesion in our study. Similar results were reported in other studies [1,9–11]. ACR was graded into mild, moderate and severe according to Rejection activity index (RAI) [12]. Mild ACR was the most common finding followed by moderate and severe ACR. Similar results were reported in other studies also [11,13]. In our study earliest and late ACR were reported at 7th and 980th day posttransplant respectively. Factors determining the incidence of ACR include type of immunosuppression, perioperative factors (ischaemia, infections) and donor characteristics (including age, cadaveric versus living, etc) [3,14–19].
CR is characterized by presence of ductopenia and foamy cell arteriopathy. In our study, the incidence of CR was 3.57%. Other authors have also reported the incidence of CR as 3% in liver allograft recipients [20,21]. Reasons for low incidence of CR are the unique immunologic properties of the liver and better recognition and control of acute rejection, but they may also be related to the remarkable regenerative capabilities [22,23]. The occurrence of CR is due to repeated ACR, CMV infection, high donor age, long cold ischaemic period and inadequate/suboptimum immunosuppression or poor compliance [3,20,24].
PRI occurs during organ harvesting. The factors responsible for PRI include donor and recipient hypotension, warm/cold ischemia and reperfusion injury [3]. In our study PRI was observed in 17.8% cases within 5-56 days post-transplant. It varies from 7-26 % in different published studies [1,10,25]. In mild PRI predominant features are microvesicular steatosis, mid zone 3 hepatocellular swelling, canalicular cholestasis and in severe injury centrilobular hepatocellular swelling, necrosis and cholestasis [1].
Hepatitis C recurrence is nearly universal after transplantation. The posttransplant course of hepatitis C is associated with a more rapid progression of fibrosis than in the native liver, with the development of cirrhosis after 5 years in 28% of cases [26]. Samuel D et al., mentioned that early recognition and intervention of recipients with rapidly evolving recurrent hepatitis C following orthotopic liver transplantation (OLT) is the only practical approach to improve outcome of these patients [27]. HCV recurrence was reported 66.67% in present study. Out of 3 patients of HCV, 2 patients developed HCV recurrence. Drug toxicity was reported in 8 (14.29%) cases. The biopsy findings included mainly microvesicular steatosis and cholestasis. The main therapeutic drugs were corticosteroids, azathioprine and cyclosporine/tacrolimus. Similar findings were reported by others [1,28]. The antibiotics such as sulfamethoxazole-trimethoprime and amoxicillin in patients after liver transplantation are also associated with drug-induced cholestasis [10,29,30].
One case of acute cholangitis secondary to hepatic artery thrombosis (HAT) on 27 days post transplant was also reported. Yu YY et al has mentioned that intrahepatic biliary injury (IBI) is characterized by non-anastomotic biliary strictures and is a relatively late complication, usually diagnosed between 1 and 4 months after liver transplantation [1]. IBI is associated with ischemia, secondary to HAT, ABO incompatible blood group donors, and chronic ductopenic rejection as well as prolonged warm or cold ischemic time prior to implantation [1,31–34].
Conclusion
Alcoholic cirrhosis was the most common indication of liver transplantation in our centre. ACR and PRI were the major complication in early liver allograft dysfunction.
To our knowledge this is the first study of its kind in India and will be helpful for framing of therapeutic guidelines. It is our initial experience of four years and requires further study to know the prevalence of chronic rejection and recurrence of disease in liver transplantation.