In assisted reproductive technology (ART), the diagnosis of male infertility has been conducted based on the assessment and analysis of sperm concentration, motility and morphology with the aim of obtaining the best quality of spermatozoa. Any type of damage present in sperm DNA can lead to ART failure [1]. Sperm DNA fragmentation might be the most frequent cause of paternal DNA anomaly transmitted to progeny, as it is found in a high percentage of spermatozoa in subfertile and infertile men; such fragmentation is negatively correlated with semen quality [1].
Sperm DNA fragmentation (SDF) is being recognized increasingly as a critical issue for male infertility. However, there are only a few techniques to identify this in routine laboratory use. Popular techniques for selecting good spermatozoa for intracytoplasmic sperm injection (ICSI) include a swim-up from either raw semen or a washed pellet, and density-gradient centrifugation [2,3]. These techniques have been used to separate functional spermatozoa suitable for use in conventional in vitro fertilization (IVF) and ICSI [4]. However, single or multiple centrifugation steps damage spermatozoa via the generation of reactive oxygen species (ROS) [5,6]. SDF can be induced by various factors such as apoptosis, ROS, chemotherapy and radiotherapy, activation of caspases and endonucleases, and by environmental toxicants [7]. SDF produces adverse consequences during postimplantation development of the embryo rather than before it [8]. Therefore, selecting spermatozoa without SDF might be essential for ART success.
Materials and Methods
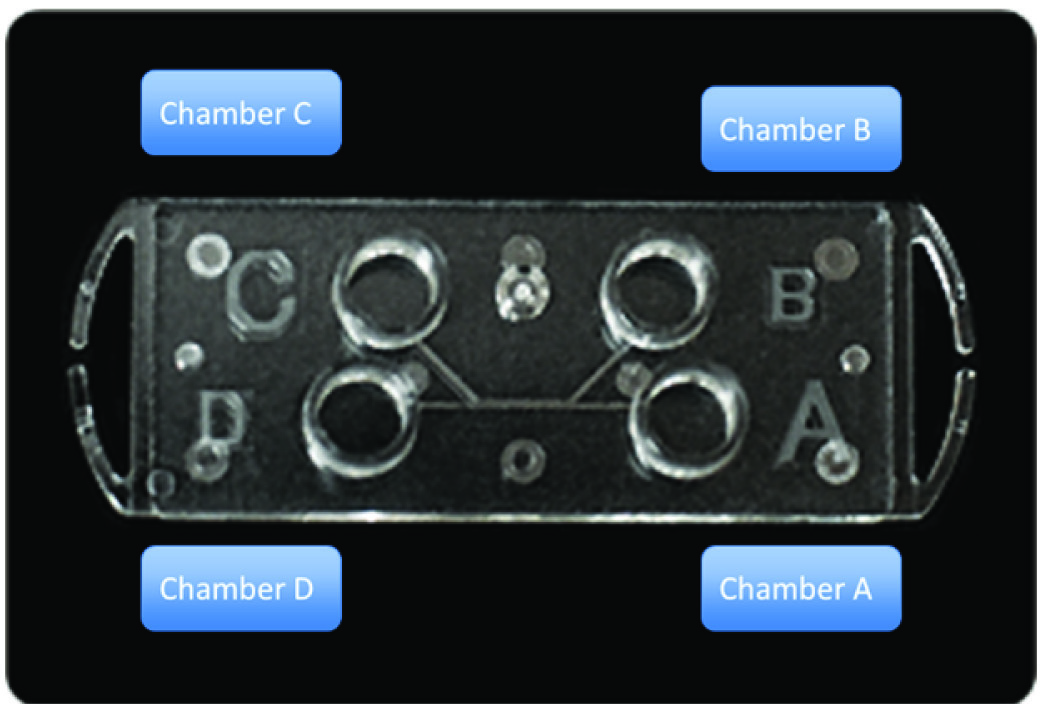
The Sperm Sorter Qualis® [Table/Fig-1] is a commercially available device based on microfluidic theory and is designed to have a channel in which nonmotile sperm and debris are obliged to flow along the initial stream and arrive at a chamber, D. Motile spermatozoa migrate to another channel, overcome the boundary of two streams and accumulate at a collecting chamber, C. Fresh semen samples obtained from 10 men (mean age 37.4 years, range 30–48; [Table/Fig-2]) with normal, oligozoospermia and asthenozoospermia were split into two groups and sorted either using Sperm Sorter Qualis® or by swim-up. Routine semen analyses were performed according to the World Health Organization (WHO) guidelines [13].
Illustration of the microfluidic device, Sperm Sorter Qualis®. An aliquot of semen is loaded into chamber A at room temperature, whereas medium without semen is loaded into chamber B. Spermatozoa with good quality are retrieved from chamber C
Semen analysis of samples in initial samplesq
| Sprem numbers (x106) | Motility (%) | Abnormal Morphology (%) | Linearity (%) |
|---|
| 1 | 83 | 53 | 30 | 60 |
| 2 | 75 | 72 | 30 | 70 |
| 3 | 57 | 61 | 25 | 60 |
| 4 | 7.4 | 46 | 30 | 50 |
| 5 | 13.6 | 41 | 60 | 50 |
| 6 | 3.2 | 94 | 30 | 50 |
| 7 | 37 | 32 | 25 | 60 |
| 8 | 19 | 37 | 30 | 55 |
| 9 | 43 | 26 | 35 | 60 |
| 10 | 52.5 | 20 | 45 | 45 |
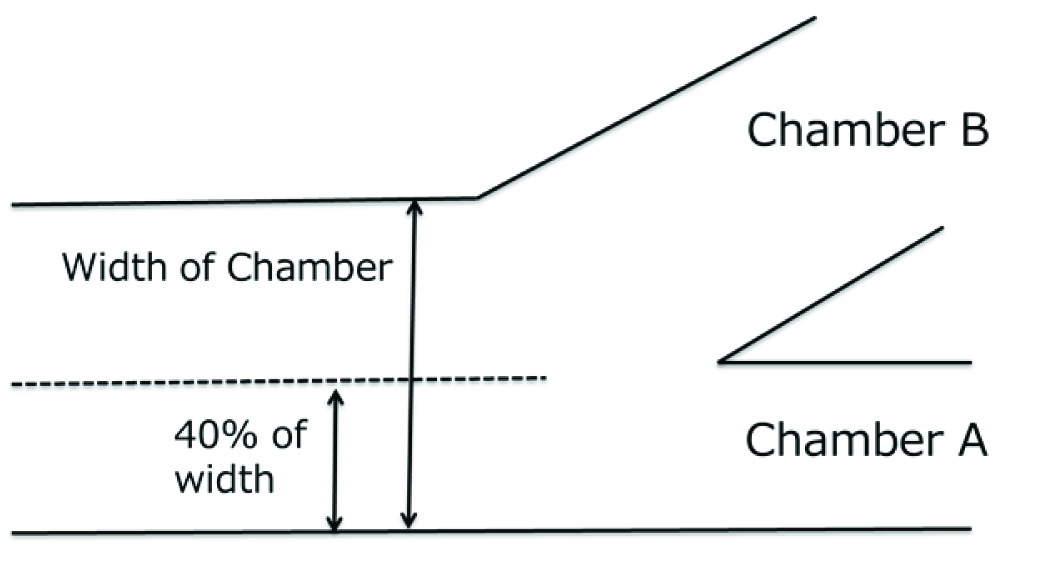
For using the Sperm Sorter Qualis®, semen samples were diluted 1:1in Universal IVF Medium® (Origio, Malov, Denmark), filtered to remove debris and then kept at 37°C until used. The device was fixed on the bottom of a 60-mm plastic dish, and 100 μl of washing medium was pipette into chambers A,B,C and D, and retrieved. Then 65μl aliquots of diluted spermatozoa were loaded into chamber A at room temperature [Table/Fig-1]; 100 μl of medium without semen was loaded into chamber B, and 20 μl of medium was loaded into each of chambers C and D. The volume of medium in chamber B was adjusted to be 40% of the whole width of the device [Table/Fig-3]. Spermatozoa in the medium in chamber C were retrieved 30 min after samples had been loaded and subjected to analysis and examination of DNA fragmentation.
This illustration shows adjustment of liquid volume in laminar flow. Volume of medium in chamber B is adjusted to 40% width to the whole chamber width
A separate swim-up method was performed as follows [6]. Briefly, diluted semen was layered onto 50% Percoll® (GE Healthcare Life Sciences, Pittsburg, PA, USA), 50% Universal IVF medium® and centrifuged for 20 min at 652g at room temperature. The supernatant was removed and pellet was resuspended with 1 ml of medium, and centrifuged for 5 min at 163g. The pellet was used for swim-up between 5 to 30 min after removing the supernatant and debris.
SDF was assessed using Halosperm® kits (Halotech DNA SL, Madrid, Spain) according to the instructions of the company. Glass slides with samples were stained with Diff Quik® (Dade Behring, Deerfield, IL, USA) and 300 spermatozoa per slide were observed in each sample for evaluating SDF. SDF was shown as the percentage.
Statistical Analysis
Data are shown as the mean±SD. Parametric data were analyzed statistically using Student’s t-tests. Paired tests were used for comparing data before and after sperm separation and non-paired tests were used for comparing the Sperm Sorter Qualis® and swim-up group. The software used for statistical analysis was StatMateIII (Human Body, Tokyo, Japan) and p<0.05 was considered significant.
Results
The initial sperm counts from three of the subjects were more than 50×106/ml with high motility [Table/Fig-2]. For three others, the counts were less than 15×106/ml with high motility of over 40%, and the remaining four men had counts of more than 15×106/ml with motilityof less than 40%. The rates of morphological abnormalities and linearity of sperm movement were similar in these three groups.
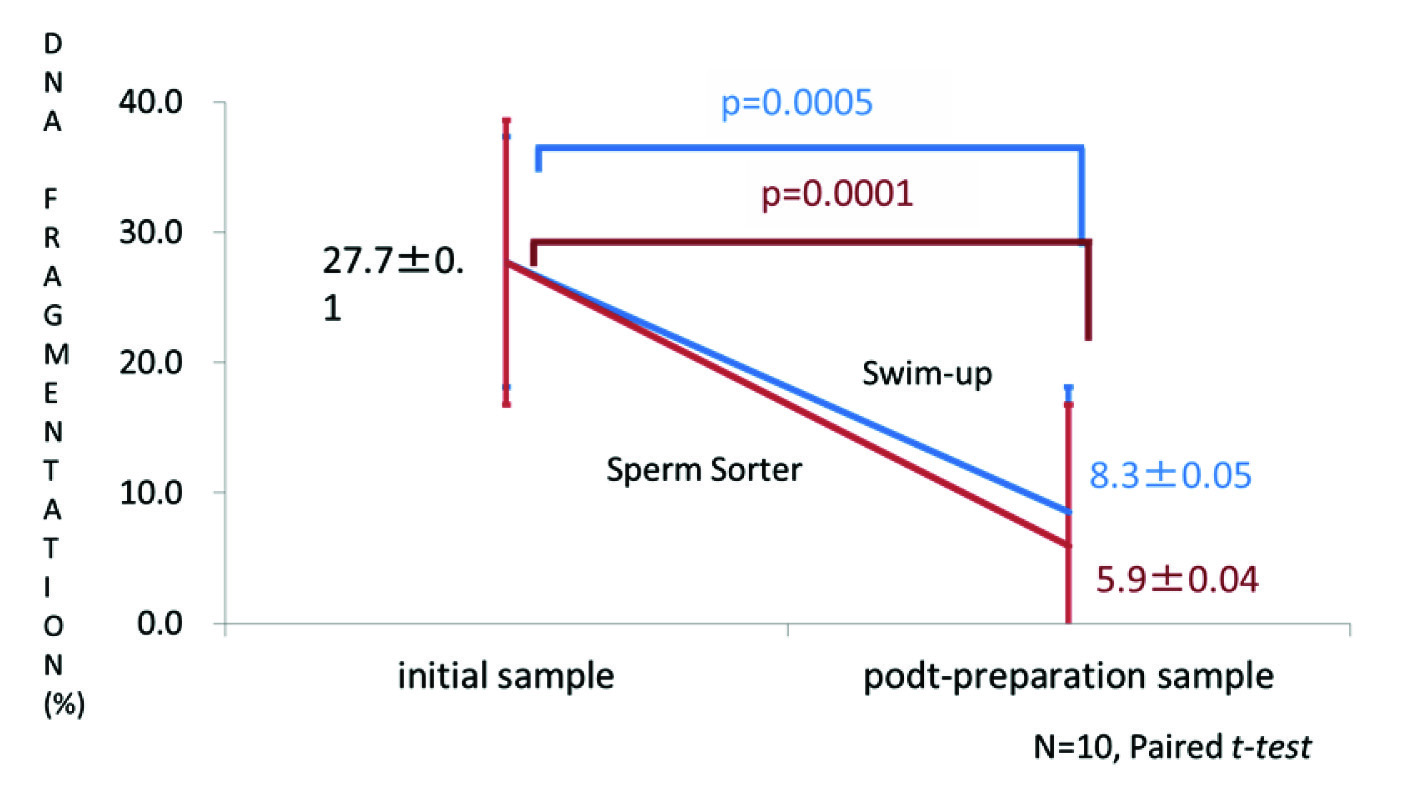
The percentage of SDF in the initial semen samples was 27.7% and in the post-centrifugation samples it was equivalent to the initial samples (25.8%; [Table/Fig-4]). SDF decreased to 8.3% after swim-up preparation (paired t-test, p=0.0005; [Table/Fig-4]) and to 5.9% in samples sorted by the Sperm Sorter Qualis® (paired t-test, p=0.0001). Similar findings were evident when DNA fragmentation of normal individuals was compared with a low motility group and a low sperm count group (data not shown).
The percentage of sperm DNA fragmentation (SDF) before and after preparation by swim-up or Sperm Sorter Qualis®. The percentage of SDF decreased significantly with both techniques
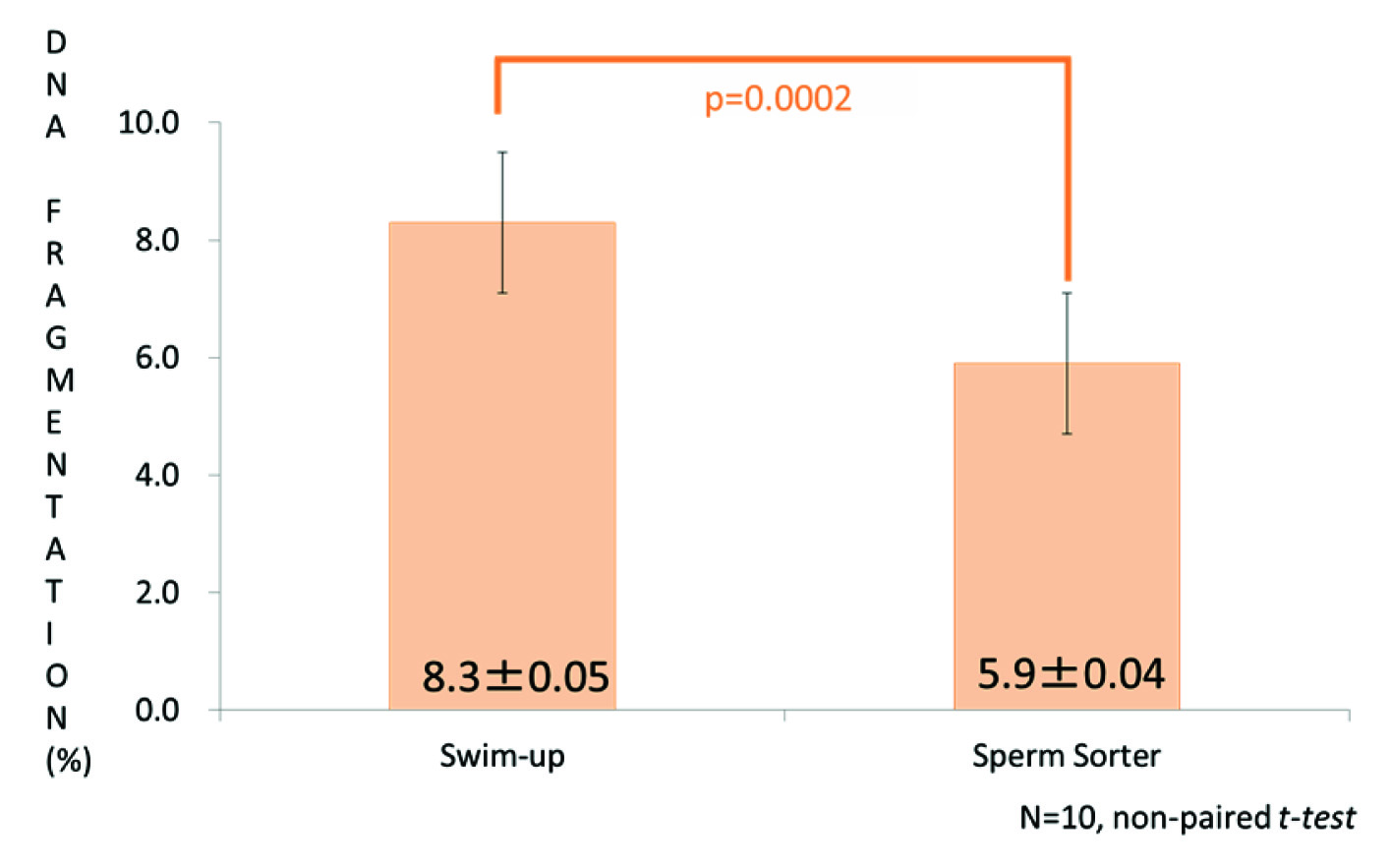
When the percentage of SDF was compared between the swim-up and Sperm Sorter Qualis® results, that of the microfluidic device was significantly lower than swim-up (nonpaired t-test, p=0.0002, [Table/Fig-5]).
Comparison of SDF percentages between the swim-up and Sperm Sorter Qualis® preparations. That in the latter group was significantly lower than after swim-up
Discussion
We found that motile spermatozoa prepared by the Sperm Sorter Qualis® showed reduced SDF compared with presorted samples and samples prepared by swim-up, which is a common method for sperm selection in ART.
Selection of the best spermatozoa and elimination of damaged spermatozoa are critical for successful IVF and ICSI in infertility clinics. At the present time, the most prevalent methods for isolating good spermatozoa are density gradient separation by the Percoll® and swim-up methods. However, one or two centrifugation steps are required to separate spermatozoa in both methods, and this might contribute to damage [14]. Furthermore, sperm damage by centrifugation might lead to increased levels of ROS causing SDF [15]. However, this microfluidic device is able to select good spermatozoa without centrifugation [10]. This should cause much less stress to spermatozoa leading to the selection of good quality spermatozoa. The advantages of this device are that it can select highly motile and morphologically normal spermatozoa from semen samples and because they show less SDF for use in ART procedures [10–12].
SDF can impair fertility for couples using ART. Mechanisms generating this defect in semen include abortive apoptosis, defective maturation and oxidative stress caused by ROS production [1]. Among these mechanisms, SDF occurs frequently during centrifugation as an iatrogenic problem during the handling of samples and exposure to oxygen [5,14]. However, the impact of SDF on clinical outcomes of ART is still unclear, so it is difficult to decide whether testing for SDF should be carried out routinely as part of infertility treatments.
Techniques for determining SDF include the sperm chromatin structure assay (SCSA), terminal deoxynucleotidyl-mediated fluorescein-dUTP nick-end labeling (TUNEL) for apoptosis, in situ nick translation, the single-cell gel electrophoresis assay, acridine orange DNA staining, and the sperm chromatin dispersion test (Halosperm®) [1]. The results obtained by TUNEL and SCSA are occasionally inconsistent [16], so there is no obvious consensus as to which assay method is best correlated with the successful pregnancy rate in ART [1]. Although SCSA is considered to be a precise and repeatable test to detect SDF [17], ART clinics need access to expensive flow cytometry apparatus and specific software for detecting SDF. Therefore, we chose the The Halosperm® to assess SDF in sperm in this study because it can be performed simply using a light microscope. The Halosperm® is a standardized kit for applying sperm chromatin dispersion assays to measure SDF [18].
The general prevalence of SDF varies between studies and detection methods. Belloc et al., surveyed 1974 normozoospermic semen samples and 4345 samples from infertile men, and the cutoff value of >30% SDF was significantly more prevalent in the infertile group [19]. In the normozoospermic group, the mean SDF value was 17.6%. Evenson et al., suggested that an SDF value of 25% increases the time taken to achieve a natural pregnancy, reduces the odds of pregnancy following intrauterine insemination, increases the miscarriage rate or can indicate complete male infertility [17]. Avendano assessed the prevalence of DNA fragmentation as TUNEL-positive spermatozoa with a rate of 3.9% in a fertile group of men versus 9.8% in a subfertile group [20].
In the present study, 27.7% of spermatozoa in the initial semen samples showed SDF. This general prevalence of SDF in normospermic men is not meaningful, as previous studies separated groups into two according to SDF rates by <10% to >35% to analyze the influence of SDF on pregnancy [21–23]. However, the SDF value of 27.7% in the initial samples of this study is likely to be within the normal range.
Conclusion
From this study, as the incidence of SDF was lower when sperm sorting was performed by the microfluidic device than by a conventional swim-up technique, this method is expected to be able to select good quality spermatozoa for ICSI without further damage caused by additional testing in an ART setting.
Ethical Approval
This study was approved by the Research Committee of Hanbusa Women’ Clinic and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
All patients gave their informed consent prior to their inclusion in the study allowing the use of their data for research purposes.