Antibiotics are the key drugs for treatment of infections and are among the most commonly prescribed drugs in neonatal intensive care unit (NICU) [1]. Worldwide population consists of about 28% children and infants who are most susceptible to infective diseases due to under developed immune system [2]. Several studies have reported that 50%-85% of children receive antibiotics prescribed by physicians [3]. In addition, neonates are among the most vulnerable population groups to contract infections [4] and are also prone to harmful effects of drugs due to differences in pharmacodynamic and pharmacokinetic characterstics [5].
The use of antimicrobial agents, especially antibiotics has become a routine practice for the treatment of neonatal illnesses [6]. Antibiotic guidelines are standard set of guidelines for the treatment of infectious diseases based on local culture sensitivity data. These guidelines help the physician to prescribe the antibiotics rationally to paediatric patients when definitely indicated (WHO model formulary for children, 2010) [7]. In spite of these, neonates are at high risk for opportunistic or nosocomial infections due to prolonged hospitalization and immunosuppressed condition [8]. An overall rise in health care costs, lack of uniformity in drug prescribing and the emergence of antibiotic resistance, are issues of monitoring and control of antibiotic use is of growing concern [9].
Thus, judicious use of antibiotic is an important way to reduce the problem of antimicrobial resistance. Hence, detailed rational knowledge of antibiotic prescribing pattern must be implemented in clinical practice. Neonates require more attention while prescribing antibiotics in order to avoid the drug resistance, adverse drug reactions and drug-drug interactions. Moreover, antibiotics are the class of drugs commonly prescribed in the NICU. Thus, the aim of our study was to observe and analyse the prescribing pattern of antibiotics in NICU of a tertiary care hospital in Western Maharashtra, India.
Materials and Methods
An observational and prospective study of six months duration was carried out in the NICU of a tertiary care hospital in Pune from April to September 2014. All the 528 neonates admitted to the NICU for more than 24 hours during the study period were included after the Institutional Ethics Committee approval. Neonates died or discharged within 24 hours of admission were excluded from the study. Study was carried out by evaluating medication records of admitted neonates. Data pertaining to gestational age, birth weight, gender, diagnosis, and patient outcome as percentages of survival, discharge and death were recorded. Details of antibiotics administered to each patient were recorded.
Statistical Analysis
The results were presented as percentage for categorical variables and mean±standard deviation for continuous variables. Chi-square test or Fisher’s exact was used to compare categorical data
Results
A total of 528 neonates were included in the study with male preponderance (62%) and out born referrals, 64.2%. Among 528 admitted neonates, 246 (46.6%) were term and 282 (53.4%) were preterm. The mean gestational age was 35 weeks (SD ± 3.2, minimum= 23, maximum = 42). The mean birth weight was 2.0 kg. (SD ± 0.7, minimum 0.5 kg, maximum 4.0 kg). Twenty four deaths were recorded during study period. Neonatal jaundice, pneumonia, neonatal sepsis, perinatal asphyxia, prematurity were the common indications for admission in NICU [Table/Fig-1].
Disease pattern and patients’ outcome
| Disease | Survival(%) | Death | DOR | DAMA | Chi2 test value (df)p-value |
|---|
| Neonatal Jaundice | 191(88.8) | 5(2.3) | 4(1.7) | 15(7.0) | 4.3 (3)p = 0.224 |
| Neonatal Sepsis | 55(70.51) | 12(15.4)@ | 1(1.3) | 10(12.8)@ | 30.5 (3)p < 0.001 |
| Respiratory distress syndrome(RDS) | 47(73.4) | 11(17.2)@ | 1(1.5) | 5(7.8) | 27.1 (3)p < 0.001 |
| Perinatal Asphyxia | 64(92.8) | 1(1.5) | 0(0.0) | 4(5.8) | 3.9 (3)p = 0.372 |
| Pneumonia | 91(85.1) | 6(5.6) | 2(1.9) | 8(7.5) | 0.40 (3)p = 0.921 |
@ Higher death rate was observed in these patients as compared to other patients
p<0.01using Chi-square test. Values in parenthesis are in percentages
Survival rate was highest in perinatal asphyxia, neonatal jaundice and pneumonia. Significanly more number of deaths were observed in the neonates with neonatal RDS and neonatal sepsis (p<0.05).
A total of 1123 antibiotics have been prescribed to 370 neonates. The majority of neonates 207 (55.9%) received between 1- 2 antibiotics, 138 neonates (37.3%) had 3 to 5 antibiotics prescribed, while 25 (6.7%) neonates were prescribed more than 5 antibiotics [Table/Fig-2].
Number of antibiotics received by the neonates
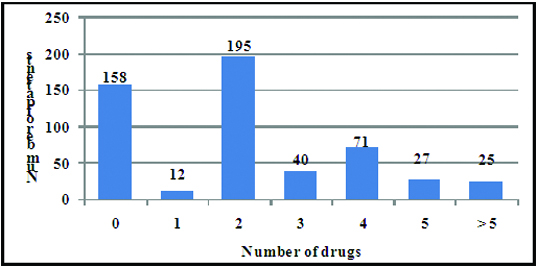
[Table/Fig-3] shows that more antibiotics were used in very preterm neonates with stastically significant negative association for number of antibiotics used with gestational age.
Number of antibiotics and gestational age
| No. Antibiotics received | Gestational age | Totaln=528 | χ2 Value (df) | p-value |
|---|
| T | M | F | P |
|---|
| 0 | 0 | 2 | 66* | 84* | 152 | 61.33 (9) | <0.001 |
| (0) | (3.92) | (31.73) | (34.15) |
| 1 - 2 | 4 | 12 | 85* | 106* | 207 |
| (26.67) | (23.53) | (40.87) | (43.09) |
| 3 - 5 | 3 | 27* | 57 | 51 | 138 |
| (20) | (52.94) | (27.40) | (20.73) |
| > 5 | 8 | 10* | 8 | 5 | 31 |
| (43.33) | (19.61) | (3.85) | (2.03) |
n= 528; * p<0.001 using chi square test. Values in parenthesis expressed in percentages
According to the WHO ATC classification system 2013 (16th edition accessed on 19th October 2014) anti- infectives for systemic use (67.7%) have been most freqently prescribed, followed by drugs for the respiratory system (n= 157) and nervous system (n= 149) [Table/Fig-4].
Number of drug prescriptions in NICU according to WHO ATC Classification [10]
| Drugs | No. of prescription (%) |
|---|
| Antiinfective for systemic use | 1123 (67.7) |
| Respiratory System | 157 (9.5) |
| Nervous system | 149 (9.0) |
| Cardiovascular System | 115 (6.0) |
| Alimentary tract | 52 (3.1) |
| Sytemic Hormonal preparations | 36 (2.1) |
| Others | 26 (1.6) |
| Total | 1658 |
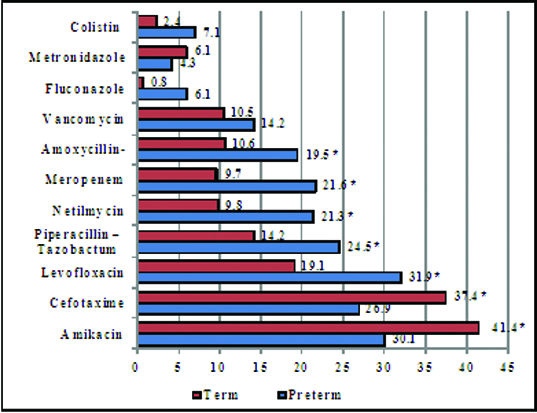
Among antibiotics Amikacin (n =187), Cefotaxime (n= 168) and Levofloxacin (n= 137) were the drugs most often prescribed [Table/Fig-5]. Antibiotic prescribing trends amongst preterm and term neonates revealed that there was statistically significant difference between the two groups (p<0.05). Amikacin and Cefotaxime were given more to term infants whereas other antibiotics (Levofloxacin, Pipercillin- tazobactum, Meropenem) were prescribed more to preterm neonates. (p<0.05) [Table/Fig-6].
List of the most frequently used systemic antibiotics
| Antibiotics | No. of Prescriptions |
|---|
| Amikacin | 187 |
| Cefotaxime Sulbactam | 168 |
| Levofloxacin | 137 |
| Piperacillin – Tazobactam | 112 |
| Netilmycin | 89 |
| Meropenem | 84 |
| Amoxycillin-Clavulanic Acid | 81 |
| Vancomycin | 66 |
| Fluconazole | 30 |
| Metronidazole | 27 |
| Colistin | 26 |
Antibiotics use with respect to gestational age
* p < 0.05 using chi square test
Amikacin and cefotaxime was prescribed to significantly more (p<0.05) number of outborn patients whereas Netilmycin and Amoxycillin-Clavulanic Acid were given more to inborn neonates (p<0.05). Along with these drugs, outborn sick patients also received Piperacillin-tazobactum, Meropenem, Vancomycin (p<0.05) [Table/Fig-7].
Antibiotics use in inborn and outborn neonates
| Drug | Inborn(%)n=189 | Outborn (%)n=339 | Total (%)n=528 | χ2 test values(df) |
|---|
| Amikacin | 7.9 | 50.7# | 35.4 | 97.2 (1)p<0.001 |
| Cefotaxime | 6.8 | 45.7# | 31.8 | 84.4 (1)p <0.001 |
| Levofloxacin | 21.7 | 28.3 | 25.9 | 2.8 (1)p = 0.93 NS |
| Piperacillin– Tazobactam | 13.2 | 23.3# | 19.7 | 7.7 (1)p = 0.005 |
| Netilmycin | 39.6# | 2.6 | 15.9 | 127.6 (1)p < 0.001 |
| Meropenem | 11.6 | 18.5# | 16.1 | 5.1 (1)p = 0.024 |
| Amoxycilin-clavulanic acid | 38.1# | 2.6 | 15.3 | 108.2 (1)p <0.001 |
| Vancomycin | 6.9 | 15.6# | 12.5 | 8.5 (1)p = 0.004 |
| Fluconazole | 1.6 | 4.7 | 3.4 | 3.4 (1)p = 0.064 NS |
| Metronidazole | 2.1 | 6.7# | 5.1 | 5.4 (1)p = 0.020 |
| Colistin | 2.6 | 6.1 | 4.9 | 3.2 (1)p = 0.071 NS |
# p<0.05 using chi square test
Discussion
This study found that antibiotics were the most commonly prescribed drug class in neonates in a tertiary level NICU. The main aim of this study was to evaulate the prescribing pattern of antibiotics The majority of antibiotics showed highest exposure rates in infants with lower gestational age (<32 weeks) probably because of poor immunity, prolonged hospital stay and high risk of sepsis. [Table/Fig-2] shows 55.94% patients received between 1 to 2 antimicrobials, 30% patients had 3 to 5 antimicrobials prescribed, while 14.05% patients received 5 and above antimicrobials. Neubert et al., observed that 90.7% of all study patients were treated with antibiotics & mostly two antibiotics [11]. Within the group of very preterm infants 96.2% patients were treated with antibiotics [11]. These absolute numbers are in line with the data published from another hospital in Germany by Gortner et al., where the exposure to antibiotics in preterm neonates (24–29 weeks’ gestation) was 98.8% [12]. Similar high exposure numbers for antibiotics are reported from the USA & Italy [13,14]. The high rate of antibiotic exposure in our study is similar to previous studies carried out in India as well as other countries and is probably due to the standard practice of administering antibiotics pending bacterial culture results in sick neonates and is not a true reflection of the incidence of bacterial infection [15–17]. A study by Fonseca et al., revealed that the highest use was in neonates with birth weight less than 1500 g and 92% of them received antibiotics within the first 48 hours after birth [18]. These findings may be explained by high susceptibility of neonates to bacterial infections, especially for very preterm neonates, who need intubation and mechanical ventilation. Clinical findings are usually variable, laboratory test (i.e. white blood cell count, C-reactive protein) are not highly sensitive and specific during the first hours following birth [19] which also accounts for the high rate of antibiotics treatment of neonates. As deleterious infections often become manifest without obvious clinical and laboratory signs, antibiotic treatment is indicated in sick neonates even if the clinical signs are only minimal. Among antimicrobials, Amikacin, Cefotaxime and Levofloxacin were the drugs most often prescribed. Amikacin and Cefotaxime were given more to term infants whereas higher antibiotics, Levofloxacin, Piperacillin-tazobactum and Meropenem were prescribed more to preterm neonates. Use of higher antibiotics mainly Levofloxacin, Vancomycin, Fluconazole, Metronidazole and Colistin was significantly more in patients with extreme preterm and very preterm babies. Amikacin, cefotaxime were most frequently used antimicrobials in previous Indian studies [16,20]. However, looking at individual drugs a great variation with regard to which drugs are used most frequently is apparent [11,13].
This heterogeneity indicates that empiric antibiotic treatment varies among different countries and in NICUs of the same country. This is because currently no standard guidelines regarding the choice of empiric antibiotics exist. This finding is not surprising as; Cochrane review comparing the antibiotic regimens for suspected late onset sepsis in newborn infants concluded that there is inadequate evidence from randomized trials in favour of any particular antibiotic regimen for the treatment of suspected late onset neonatal sepsis [21]. The choice of antibiotic regimes depends upon information regarding known flora prevalent in a unit, personal experience of neonatologist and culture reports rather than being guided by comparative clinical studies. Therefore, use of individual antibiotics could not be compared with other studies, as its use in respect to gestational age groups & outborn- inborn groups was not mentioned [13,20,22]. Decisions about how to prevent neonatal sepsis, who and how long to treat, and which antibiotics to use remain important clinical problems [23,24]. So, early sepsis evaluations and empiric antibiotic treatment is required [25]. In the present study, to give wider coverage, according to NICU antimicrobial policy, the out born neonates received Amikacin & cefotaxime as an empirical therapy, while Netilmycin & Amoxicillin- clavulanic acid was common among inborn neonates. This covers both gram positive and gram negative organisms. The microbial culture positive cases showed that the main organisms were Coagulase negative Staphylococcus, Enterococcus fecalis, Staphylococcus aureus. Antibiotics were changed to Levofloxacin, Piperacillin, Meropenem or Colistin according to culture and sensitivity reports. Since in our study, neonatal sepsis was predominantly present in preterm infants. So use of these higher antibiotics was significantly (p<0.001) more in preterm babies than term patients.
Limitations
Few limitations of our study are, as study comprised most of the outborn population, drug history mainly antibiotics received by these neonates before admission could not be collected. Another limitation of our study is that data pertaining to drugs received by mother just prior or during delivery could not be revealed. This could have impact on antibiotic prescribing pattern, as symptomatic baby can have a false negative blood culture if antibiotics are given prenatally to the mother.
Conclusion
In present study, 28% of NICU patients were managed without any drug, 70.7% of neonates had received the antibiotic prescriptions and the maximum numbers of antibiotic prescriptions were found in the preterm and out born patients. The use of higher antibiotics was also more in out born sick infants. In spite of complicated neonates getting admitted to NICU, the number of drugs per prescription was not very high. Problems existed in more number of antibiotics prescribed, less use of generic drugs, the absence of the latest National essential list of medicines and high use of antimicrobial agents, not in line with culture reports. Present study suggests that Antibiotics policy to be framed & periodically reviewed: to reduce unnecessary use of antibiotics and associated problems.
@ Higher death rate was observed in these patients as compared to other patients
p<0.01using Chi-square test. Values in parenthesis are in percentages
n= 528; * p<0.001 using chi square test. Values in parenthesis expressed in percentages
# p<0.05 using chi square test