Global burden of epilepsy is approximately 0.5% affecting predominantly early childhood and late adulthood resulting in psychological and social consequences [1–3]. Unfortunately among the 5.5 million of the affected Indians only one fourth of rural population is treated [4]. The reason for the lacunae was augmented as ignorance, negligence and poor compliance [5]. WHO, the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) are carrying out a global campaign, ‘Out of the Shadows’ to increase the awareness about epilepsy by educating the people about the same [6]. Even though many conventional and newer drugs are available for antiseizure management, they are associated with several adverse effects which lead to poor compliance. Moreover they offer only symptomatic relief from seizure but not cure epilepsy [7,8]. Thus, there is an ever-increasing need for research into the pathophysiology of epilepsy and the development of newer drugs for treating epileptic seizures.
Few dihydropyridine (DHP) calcium channel blocker (CCB) had already been proven to possess anticonvulsant activity against various animal models of epilepsy [9–11]. The disadvantage with most of them is their adverse reactions and shorter duration of action. But an ideal drug for the management of epilepsy should preferably be longer acting with minimal adverse effects. Lercanidipine, a newer generation DHP CCB, is used for treatment of mild to moderate hypertension. It differs from other DHP by its unique properties like high flexibility, lipophilicity, longer duration of action and tissue selectivity [12]. Literature review also provided insights about the stable pharmacokinetics, minimal adverse effects, better tolerability and safety profile exhibited by lercanidipine [13–15]. Also, lercanidipine was found to have predominant effects on coronary vessels and also been augmented to cross the blood brain barrier [16].
To our knowledge, no studies had been done to explore its neuroprotective effect. An ideal antiepileptic should also be neuroprotective. Rotarod test and actophotometer test were routinely employed to assess the neuroprotective effect of the drug in experimental animals. Latency of fall is typically used as a quantitative endpoint to evaluate the motor function whereas locomotor activity is evaluated by actophotometer test.
Materials and Methods
Ethical Statement
The animal experiment was carried out in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The study was conducted after approval from Institutional Animal Ethics Committee for a period of 6 months. Animals were handled with utmost care and all efforts were taken to minimize their suffering.
Experimental Animals
Healthy male Swiss albino strain mice (Mus musculus) (4-6-week-old, weighing 20-30 g) were procured from Tamil Nadu Veterinary and Animal Sciences University, Madhavaram Milk Colony, Chennai, Tamil Nadu, India. Animals were acclimatized to laboratory conditions for a period of 7 days in the Animal house at our institution. They were housed in groups of six in polypropylene cages bedded with paddy husk under controlled room temperature (24-270c) in a 12 hour light dark cycle and relative humidity (55 ± 5%). The mice were fed with standard pellet diet and water ad-libitum. The experiment was conducted throughout during the light period between 10.00 and 12.00 hours. All attempts were done to minimize the number of animals.
Chemicals and Drugs
Pure form of lercanidipine was obtained from Sigma Aldrich, India and fresh solution was made in 99.9% HPLC grade methanol. Appropriate vehicle was used for control animals. Fresh solutions of standard drug diphenylhydantoin sodium (Pfizer, India) and diazepam (4 mg/kg I.P.,) was made in normal saline. The dose selection was done based on the previous studies [10].
Study Design
Randomized controlled experimental study.
Sample Size
Eighty four male abino mice were utilized to study the neuroprotective effect; 36 for anticonvulsant effect; 24 for muscle relaxant activity and 24 for spontaneous locomotor activity. Mice were randomized using random tables. The data were recorded by one investigator whereas the other investigator was blinded.
Experimental Procedure
The anticonvulsant profile of lercanidipine was evaluated against MES test. Motor coordination and spontaneous locomotor activity were tested using rotarod and actophotometer apparatus respectively.
Maximal Electroshock Seizure (MES) Test
MES was induced in mice using Electroconvulsometer. MES stimuli, comprising 0.2 seconds of rectangular positive pulses (50 mA at 50 Hz; pulse width 0.4 ms) were delivered through ear-clip electrodes. Electrodes were moistened with saline solution before application. During application of stimulus animals were restrained only by hand and released at the moment of stimulation. In present study those animals that consistently exhibited the tonic hind limb extension component (THLE) on MES application after three trials on separate days without any medication were utilized. The animals were divided into six groups consisting of six animals in each group. Group 1 and 2 served as control and standard respectively. Group 3, 4, 5 and 6 were test groups. The drug received by each group was as follows:
Group 1 - Double distilled water 0.3 ml I.P., (vehicle)
Group 2- Phenytoin sodium 25mg/kg. I.P.
Group 3- Lercanidipine 1mg/kg. I.P.
Group 4- Lercanidipine 3mg/kg. I.P.
Group 5- Lercanidipine 1mg/kg. I.P. + Phenytoin sodium 12.5 mg/kg I.P.
Group 6- Lercanidipine 3mg/kg .I.P. + Phenytoin sodium 12.5 mg/kg I.P.
The drug or distilled water (vehicle) was administered intraperitoneally to each mouse 30 minutes prior to receiving an electroshock. As proposed by Castel- Branco et al., the test was considered positive if the animal exhibited tonic extensor seizure with hind limb extension more than 900 from the body and sustained for more than 3 seconds following 10 seconds after stimulation. The mice were observed for half an hour after MES induction [17,18]. The animals used were isolated and they were not reused for any other experimental purpose. The parameters that are recorded following MES application are onset of THLE, duration of THLE, duration of clonus, number of jerks, recovery time and percentage of protection.
Onset of THLE: Time (in seconds) of onset of THLE after giving the drug or vehicle.
Duration of THLE: Time duration between onset of THLE to disappearance of THLE.
Duration of clonus: From onset of clonus to regaining of normal condition.
Number of jerks: Total number of jerks the animal encountered during clonic phase.
Recovery time: Time (in seconds) taken for the animal to completely recover from tonic and clonic phase after administration of the drug or vehicle.
Assessment of Motor impairment
The effect of lercanidipine on motor coordination was evaluated using rotarod apparatus (INCO, Ambala, India). The rotarod apparatus (INCO, Ambala, India) used in our setting consisted of an elevated rod that rotated at a constant speed (16 rpm). Only mice that were able to stay on the rod for 120 seconds were selected for the study. The selected mice were divided into four groups consisting of six animals in each group.
Group 1 - vehicle treated.
Group 2- Diazepam 4mg/kg. I.P.
Group 3- Lercanidipine 1mg/kg. I.P.
Group 4- Lercanidipine 3mg/kg. I.P.
Prior to the procedure mice in each group were given training for four days for four sessions at 1 hour interval per day. On the day of procedure, mice were tested again for their ability to balance the rod for 120 seconds. The animals were then evaluated for motor coordination at 30 min after oral administration of the drugs. The time taken (in seconds) by each animal to falls off from the rod was noted [19].
Test for Locomotor Activity
The effect of test drug on locomotor activity was assessed using digital photoactometer (INCO, Ambala, India) which operated on photoelectric cells. It helps us to access the cognitive task of the animal. Every time when the animal crosses the square arena, the beam of light falling on photocell is cut off and recorded as one. The animals were divided into four groups:
Group 1 - vehicle treated
Group 2- Diazepam 4mg/kg. I.P.
Group 3- Lercanidipine 1mg/kg. I.P.
Group 4- Lercanidipine 3mg/kg. I.P.
The animals were given vehicle or drugs depending on the group. After 30 minutes, the animals were placed on the rod and the ambulatory movement of each mouse was recorded for a period of 5 minutes. The fall off time for each animal was noted and taken as grip strength [20].
Statistical Analysis
The results were expressed as mean ± standard error of the mean. The data were analysed with one-way ANOVA followed by post-hoc Dunnett t-test. The statistical test was done using Statistical Package for Social Sciences software version 16 (SPSS Inc. Chicago, USA). p <0.01 was considered statistically significant whereas p<0.001 as highly significant.
Results
Effect of Lercanidipine on Onset of Tonic Hind Limb Extension
Complete abolition of THLE was observed with the phenytoin treated group (25mg/kg) and was highly significant (p<0.001) compared to vehicle treated animals [Table/Fig-1]. Highly significant reduction in onset of THLE was obtained with combined administration of lercanidipine 1 and 3 mg/kg with standard drug (p<0.001) compared to vehicle treated group. Lercanidipine administered in a dose of 3 mg/kg had significantly (p=0.003) reduced the onset of THLE.
Effect of lercanidipine on onset of tonic hind limb extension in mice
| Group | Treatment (mg/kg, I.P.) | Onset of tonic hind limbextension(time in seconds) | Duration of clonus(time in seconds) | Number of jerks | Recovery time(time in seconds) |
|---|
| 1 | Vehicle | 3.33 ± 1.8 | 16.83 ± 6.41 | 6 ± 1.15 | 90 ± 69.70 |
| 2 | Phenytoin sodium (25) | 0** | 0** | 0** | 0.17 ± 0.37** |
| 3 | Lercanidipine (1) | 4 ± 1.549 | 4 ± 1.549** | 2.833 ± 1.471* | 32.166 ± 12.481* |
| 4 | Lercanidipine (3) | 6.83 ± 1.46* | 2.5 ± 1.76** | 0.5 ± 1.12** | 27.17 ± 12.92* |
| 5 | Lercanidipine (1) + Phenytoin sodium (12.5) | 8.5 ± 1.51** | 1.66 ± 2.25** | 0.5 ± 1.224** | 4.166 ± 1.471** |
| 6 | Lercanidipine (3) + Phenytoin sodium (12.5) | 11.5 ± 1.87** | 0.66 ± 0.816** | 0.16 ± 0.408** | 1.41 ± 1.02** |
Values were expressed as mean ± SD for all the six groups
*p<0.01; **p<0.001 as compared to vehicle treated group
Comparison was done by one-way ANOVA followed by Dunnett t-test
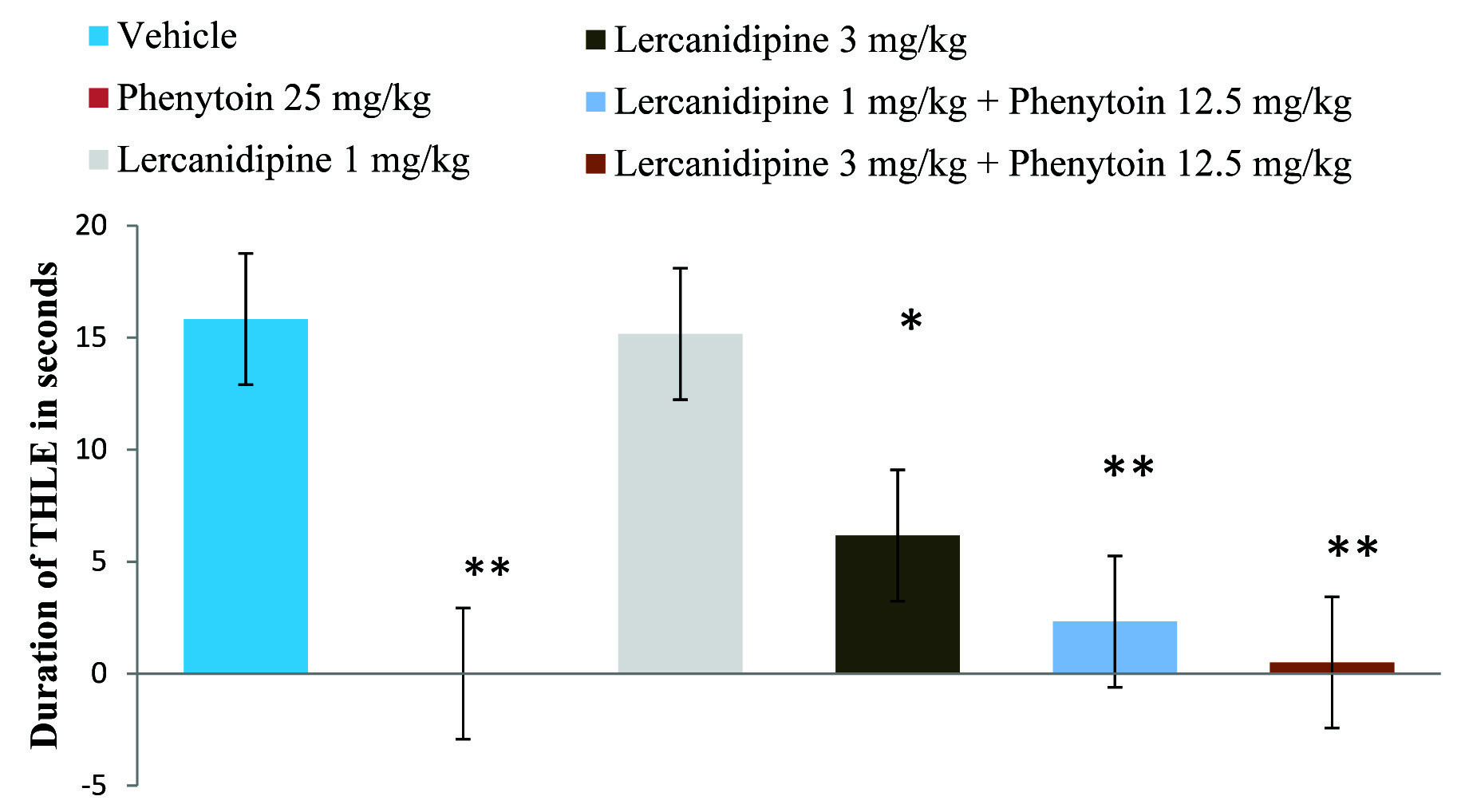
Effect of Lercanidipine on Duration of Tonic Hind Limb Extension
Compared to the control, lercanidipine 3mg/kg produced statistically significant (p<0.01) inhibition of THLE but with 1mg/kg the result was not statistically significant (p=0.999). Highly significant reduction (p<0.001) in tonic hind limb extension was observed with combined treatment of Lercanidipine (1 and 3 mg kg-1) and standard drug [Table/Fig-2].
Effect of lercanidipine on duration of tonic hind limb extension in mice
Values were expressed as mean ± SD for all the six groups
*p<0.01; **p<0.001 as compared to vehicle treated group
Comparison was done by one-way ANOVA followed by Dunnett t-test
Effect of Lercanidipine on Duration of Clonus
Complete absence of clonus was observed with standard drug therapy [Table/Fig-1]. Treatment with lercanidipine in a dose of 1 and 3 mg/kg had resulted in highly significant (p<0.001) reduction in duration of clonus compared to vehicle treated group. Moreover lercanidipine in both the doses when combined with phenytoin sodium (12.5 mg/kg) reduced the duration of clonus which was also highly significant (p<0.001).
Effect of Lercanidipine on Number of Jerks
The number of jerks observed with lercanidipine 1 mg/kg treated group was statistically significant (p<0.01) compared to vehicle treated group [Table/Fig-1]. Whereas combination treatment with lercanidipine 1 and 3mg/kg and phenytoin sodium (12.5 mg/kg) as well as lercanidipine alone at higher dose had decreased the number of jerks which were highly significant (p < 0.001) compared to vehicle treated group.
Effect of Lercanidipine on Recovery Time
In the vehicle treated animals the recovery time was prolonged whereas with phenytoin and combined treatment of lercanidipine and phenytoin at both doses, highly significant (p<0.001) reduction in recovery time was observed [Table/Fig-1]. However with test drug treated animals in doses of 1 and 3 mg/kg the recovery time was only significantly (p<0.01) reduced compared to vehicle treated group.
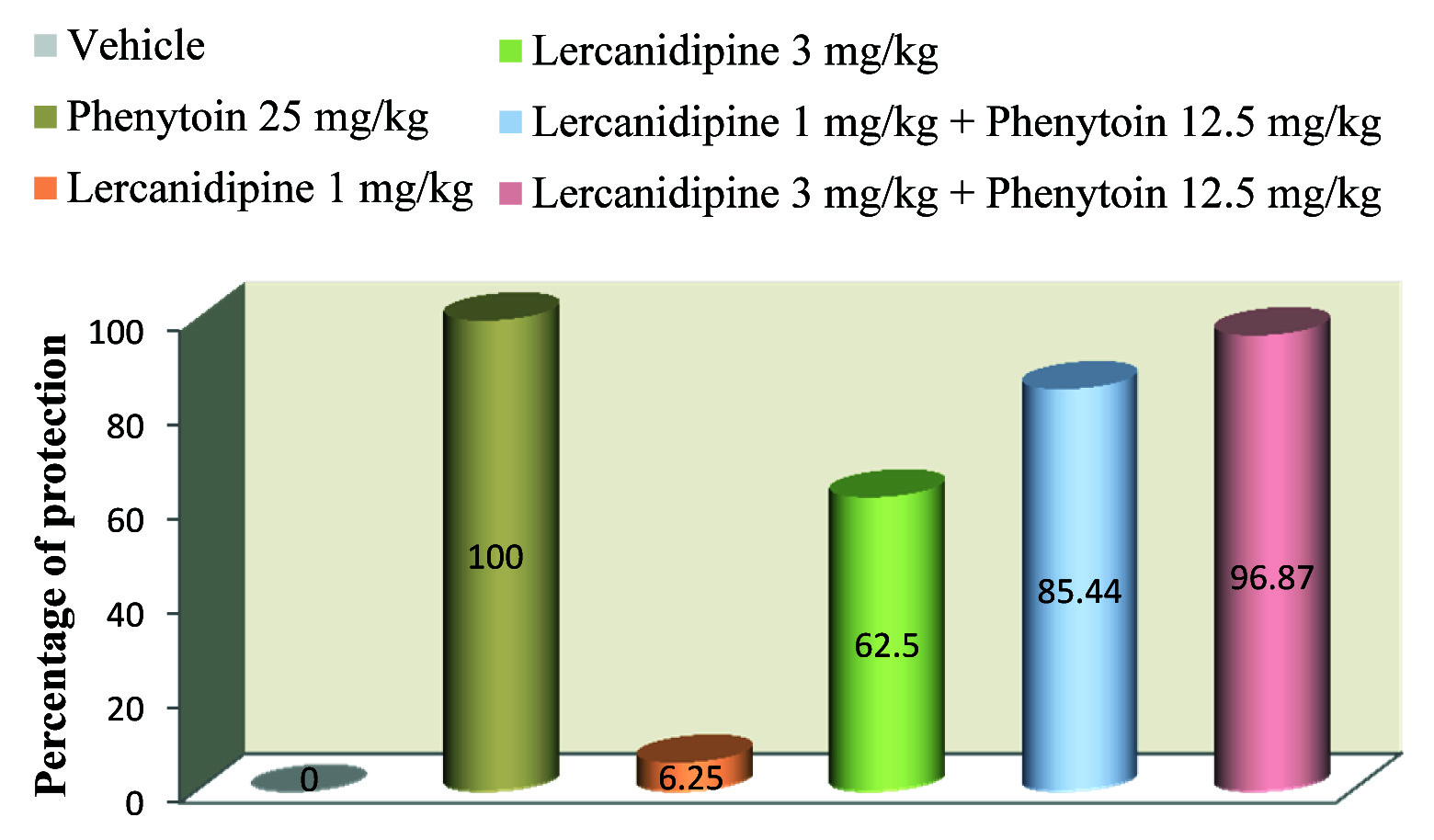
Effect of Lercanidipine on Percentage of Protection
Lercanidipine in doses of 1 and 3 mg/kg had 6.25% and 62.5% protection respectively as compared to vehicle treated group [Table/Fig-3]. Moreover lercanidipine 1 and 3 mg/kg combined with standard drug phenytoin (12.5 mg/kg) had high percentage (85.44% and 96.87% respectively) of protection against convulsion whereas 100% protection was noticed with standard group.
Effect of lercanidipine on percentage of protection in mice
Values were expressed as percentage
Effect of Lercanidipine on Motor Coordination
The mean time duration on rotating rod was significantly increased (p<0.001) with lercanidipine compared to standard group [Table/Fig-4].
Effect of lercanidipine on muscle relaxant activity.
| Group | Treatment (mg/kg, I.P.) | Fall off time (in seconds) |
|---|
| 1 | Vehicle | 263.3333 ± 23.48333## |
| 2 | Diazepam (4) | 22.66667 ± 4.966555** |
| 3 | Lercanidipine (1) | 236.1667 ± 16.90463## |
| 4 | Lercanidipine (3) | 253.6667 ± 26.81542## |
Values were expressed as mean ± SD for all the six groups
*p<0.01; **p<0.001 as compared to vehicle treated group
#p<0.01;##p<0.001 as compared to diazepam treated group
Comparison was done by one-way ANOVA followed by Dunnett t-test
Effect of Lercanidipine on Locomotor Activity
In mice treated with lercanidipine, locomotor activity was highly significant (p <0.001) compared to the standard group [Table/Fig-5].
Effect of lercanidipine on locomotor activity
| Group | Treatment(mg/kg, I.P.) | Locomotor activity count / 5 minutes |
|---|
| 1 | Vehicle | 518.5 ± 28.45171* |
| 2 | Diazepam (4) | 144.8333 ± 5.636193** |
| 3 | Lercanidipine (1) | 508.6667 ± 18.86443© |
| 4 | Lercanidipine (3) | 512.1667 ± 23.82785©© |
Values were expressed as mean ± SD for all the six groups
*p<0.01; **p<0.001 as compared to vehicle treated group
©p<0.01; ©©p<0.001 as compared to diazepam treated group
Comparison was done by one-way ANOVA followed by Dunnett t-test
Discussion
The preclinical evaluation of any compound with potential anticonvulsant activity is accomplished through a series of in-vivo and in-vitro tests [21].
In present study MES was used which is the gold standard model for generalized tonic clonic seizure. MES is highly reproducible, behavioural model and the electrographic seizures were consistent with human epilepsy. It exerts its convulsant activity by inhibiting the Gamma-Aminobutyric Acid (GABA) at GABA receptors which is the major inhibitory neurotransmitter implicated in epilepsy [17]. This model is used to identify the compounds which prevent seizure spread and is valid not only for screening the compounds which prolong the inactivated state of sodium channel but also used for screening GABA enhancing drugs and glutamate receptor antagonists. In this order calcium channel antagonists were also tried for their potential anticonvulsant effect and proven to be effective [10,11,22,23]. In our study the test compound lercanidipine which is the recent calcium channel blocker was tested against MES induced seizure in two different doses individually and combined with the standard drug phenytoin sodium.
In the present study lercanidipine at higher dose lercanidipine (3mg/kg) had produced statistically significant reduction in the duration of THLE when compared to vehicle treated animals (p=0.006). Similar study by Sathyanarayana et al., showed reduction in the THLE with amlodipine (4.33 ± 2.75) compared to vehicle (16.33 ± 0.56) against MES induced seizure [10]. Moreover combination of lercanidipine with phenytoin possessed high antiseizure activity than compared to individual doses of the test compound. Another study had proven verapamil and nifedipine had protective effect against strychnine induced convulsions [24]. According to Meyer et al., significant protection against MES by nimodipine compared to phenytoin treatment was also documented [9].
In the present study statistically significant (p= 0.003) prolongation of latency period was obtained with lercanidipine at higher dose which is in accordance with nifedipine in a study done by Brahmane et al., [25]. In addition highly significant (p<0.001) delay in onset was seen at combined treatment of lercanidipine with phenytoin sodium (12.5 mg/kg).
The percentage of mice protected with higher dose of lercanidipine (3mg/kg) in our study was comparable to that of amlodipine protection against MES [10]. Moreover lercanidipine in two doses with phenytoin had produced high protective activity as evidenced by 85.44 and 96.87 percentage respectively. This percentage of protection was definitely high when compared to lercanidipine alone treated group. Study done by Sahadevan et al., with nifedipine showed only 30% protection which is less compared to our study [26]. They also demonstrated that flunarazine had 50% protection with MES induced seizures whereas with diltiazem no protection was observed in their study. In addition the reduction in the duration of THLE was also high in our study compared to nifedipine [26]. Yet another study with cinnarazine and nifedipine had showed 50% and 41.66% respectively protection against MES which is certainly less compared to our study. However, high percentage of protection was obtained when CCB were combined with standard drugs [25]. These findings are in agreement with our report.
Another study done with isradipine on MES induced convulsions also showed significantly reduction in MES induced convulsion and also potentiated the conventional antiepileptic drug phenytoin in higher percentage (96.6) similar to our combination treatment with lercanidipine (96.87) [27].
Earlier study done in Italy had showed reduction in clonus percentage with nifedipine, nimodipine, nicardipine and nitrendipine which is comparable to the results obtained with lercanidipine [28]. Highly significant reduction (p<0.001) in the number of jerks was observed with lercanidipine combined with standard drug. This implies that drug has potentiating effect when combined with phenytoin. The study results also revealed that lercanidipine exhibited faster recovery from MES induced seizures. Moreover lercanidipine with a standard drug phenytoin had produced even faster recovery from jerks compared to vehicle treated animals as evidenced by statistical analysis (p< 0.001). A study conducted on isradipine by Devi et al., demonstrated no significant change in clonus [27].
The standard drug used in our study was phenytoin which has the most significant effect of modifying the pattern of maximal electroshock seizures by limiting the repetitive firing of action potentials which is evoked by a sustained depolarization of mouse spinal cord neurons maintained in vitro. Phenytoin exerts its antiepileptic activity by stabilizing the neuronal membrane thereby causing prolongation of the recovery of inactivated state of sodium channels and also by inhibiting post-tetanic potential due to calcium influx inhibition [5]. CCB was also postulated to decrease the epileptic depolarization of the cerebral neurons and possess the membrane stabilizing property which could be responsible for reduction of seizure episode. In addition CCB also found to block the inflow of sodium into detonated neuron and glutamate release inhibition [25]. In MES augmented effects were observed when lercanidipine was combined with phenytoin. So this study supports that combination therapy can be effective in controlling Generalized Tonic-Clonic Seizure (GTCS) as evidenced by MES model. Moreover in the present study we used low dose of phenytoin (12.5 mg/kg) in combination therapy and obtained highly significant effect thereby adverse effects due to phenytoin therapy can be minimized. More advanced electrophysiological and biochemical studies are warranted to elucidate the nature of interactions between these two drugs.
Limitations
The limitations of our study are small sample size and also study was done in one model of epilepsy. More detailed studies in various doses using different animal models followed by human trials may help to identify the active role of lercanidipine in different types of epilepsy as an add on therapy with conventional antiepileptic drugs.
Conclusion
From the present study, we conclude that lercanidipine possesses significant anticonvulsant effect. The benefit was further increased when combined with half the dose of phenytoin. Moreover, it did not affect the muscle coordination or locomotor activity in mice. Henceforth lercanidipine is a potential candidate for the treatment of convulsions due to its favorable neuroprotective effect.