An increasing number of all ceramic materials and systems are currently available for clinical use and the interest in all ceramic restorations has increased in the recent years. Successful adhesion to dental hard tissues is a fundamental requirement prior to the insertion of tooth-coloured materials [1–4]. Most modern resin cement kits contain both an adhesive (Dentin Bonding Agent {DBA}) for bonding to the tooth structure and a dual-polymerizing cement (composite) for bonding to the restoration. When bonding ceramic to tooth structure, two different interfaces need to be considered: the dentin/adhesive interface and the ceramic/cement interface [5]. Optimization of bond strength is considered at two interfaces because lower one determines the final bond strength.
Bonding to enamel has been known as being clinically very reliable. Since its introduction in 1955, the acid-etch technique has provided an ideal surface morphology as a result of the use of 37% phosphoric acid. Etching pattern is evidenced by microporosities in to which penetration of polymerizable monomers to form resin tags that provide micro mechanical retention [6,7]. Bonding to dentin is more difficult than enamel because of its tubular structure and its intrinsic wetness. Penetration of ambiphilic molecules into acid-etched dentin is the bonding mechanism of recent dentin bonding agents [8–11]. The hybrid layer is a mixed zone of polymerized resin and entangled collagen fibrils formed by penetration of the monomers and to obtain a direct contact of resin with the collagen fibers by utilising water-chasing solvents, such as ethanol or acetone [12–14].
A different philosophy is advocated by ‘self-etching primers’, which have been evaluated in several studies [15–17]. Self-etching primers condition and prime enamel and dentin simultaneously without rinsing, these materials have inability to remove the smear plugs upon conditioning due to mild acidic nature [13,18]. These materials have the ability to dissolve hydroxyapatite partially, both within the smear layer and the dentin surface, resulting in a resin-infiltrated zone with entrapped minerals [9,19]. These attempts at creating adhesion to dentin without a separate etching step may be a noteworthy alternative to the total etch technique.
The recently introduced 1-step self-etch (all-in-one) adhesives contain two liquids which are applied to tooth substrates after mixing [20–22]. The idea for using two separate bottles for these adhesives is to isolate the hydrolytically unstable acidic resin monomers from the water that is present for ionization [23]. The latest approach in simplifying bonding to enamel and dentine are the adhesives in which all the adhesive components for etching, priming and bonding are supplied in a single bottle. Hence this study was done to compare the adhesive ability and to evaluate the shear bond strength between the CAD/CAM ceramics (CEREC blocs, Sirona) and dentin using the new adhesive Xeno III (self etch), with prime and bond NT (total etch) with two different surface treatment such as <5% hydrofluoric acid and <5% hydrofluoric acid with silane coupling agent.
Materials and Methods
Sample preparation: Forty recently extracted human molars were collected, cleaned off debris with a hand scaler and stored in saline solution at room temperature. The roots of each tooth were embedded in a plastic cylinder of 40mm x 20mm filled with an autopolymerised polymethylmethacrylate resin. The crowns of the teeth were sectioned 90° to the long axis of the teeth with a diamond rotary cutting instrument of medium grit to expose the dentin surface. The dentin surface was finished using paper discs to create a very smooth surface and reduce any micromechanical interlocking that could affect the real bonding influence of adhesive cements. To expose the dentin surface to the standardised size of the metal die, a polyethylene sheet of 40μm thickness was punched and placed over the dentin surface. Dentin surface of the tooth was etched with 37% phosphoric acid (N etch, Ivoclar vivadent, India) for 15 sec, washed with water and air dried.
A stainless steel cylindrical die of 6mm x 5mm was made [Table/Fig-1]. A putty index of the metal die (Aquasil, Dentsply, India) was made. An Opti spray (CEREC) (sirona dental systems LLC) was used over the index before scanning. Then index with 6mm x 5mm was scanned [Table/Fig-2] using CEREC 3.65 software and resultant image [Table/Fig-3,4] was milled using CEREC inlab MCXL milling machine (sirona dental systems LLC). 40 samples of CEREC blocks were made. The blocks were bonded to the exposed dentin surface using 2 different bonding agents and two different surface treatments.
Line diagram of 5mm x 6mm Master die
Scanner with CEREC 3.65 software
Initial scanned image of master die using CEREC 3.65 software
Final scanned image of master die using CEREC 3.65 software
Study design: The samples were divided into 4 groups (A1, A2, B1, B2) each one formed by 10 cylinders. In A1 group 10 ceramic cylinders were treated with < 5% hydrofluoric acid (Dentsply, India) and bonded using Prime & Bond NT (Dentsply, India) and Variolink II (Ivoclar vivadent India). In the A2 group the ceramic surface was treated with < 5% hydrofluoric acid and silane coupling agent, the treated ceramic surfaces were bonded to dentin surface using Prime & Bond NT and Variolink II. In group B1 the bonding surface of ceramic were treated with < 5% hydrofluoric acid and bonded using Xeno III (Dentsply India) and Variolink II. In B2 group the bonding surfaces of ceramic were treated with < 5% hydrofluoric acid and silane coupling agent, (Dentsply, India) the treated ceramic surface was bonded to tooth structure using Xeno III and Variolink II.
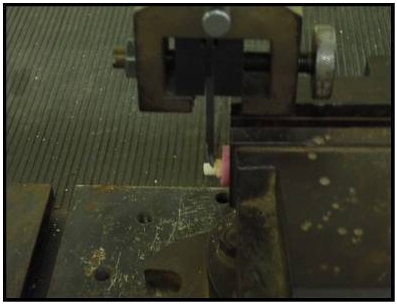
The specimens were stored in distilled water at room temperature for 24 hours after cementation. The entire study was done for a period of 3 months in Department of Prosthodontics, SRM Dental College Ramapuram. Specimens were mounted on the jig of a universal testing machine Instron 3385 with 10-KN load [Table/Fig-5], loaded at a crosshead speed of 1.0 mm/min until they are fractured and the values recorded. The calculated shear bond strength was determined by dividing, the strength at which bond failure occurred, by the bonding area.
Shear bond test using digital universal testing machine
Results
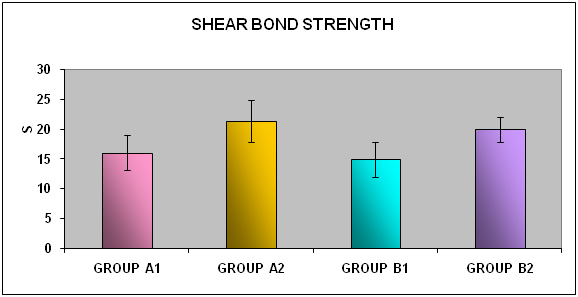
Mean and standard deviation of shear bond strength were calculated from the obtained values. The data were evaluated by student’s t-test to assess equality of mean between the paired groups (same bonding agent) and also between the unpaired groups (different bonding agent). Levene’s test was used for equality of variance between the paired and the unpaired groups. [Table/Fig-6,7] showed mean bond strength for group A1 was 16.03±2.92 MPa and A2 21.26±3.56 MPa where the p-value <0.002 showed significant difference in bond strength between A1 and A2.
DemographiMean bond strength for groups A1 & A2
| Group | Mean (MPa) | Standard deviation |
|---|
| Group A1 | 16.03 | 2.92 |
| Group A2 | 21.26 | 3.56 |
T-test for equality of means for groups A1 & A2
| Variances | Levene’s test for equality of variances | t-test for equality of means |
|---|
| F | Sig. | T | df | Sig.(2-tailed) | Mean difference |
|---|
| Equal variances assumed | 1.18 | 0.29 | -3.59 | 18 | .002 | -5.23 |
| Equal variaences not assumed | | | -3.59 | 17.33 | .002 | -5.23 |
[Table/Fig-8,9] showed mean bond strength for group B1 was 14.91±2.99 MPa and B2 19.92±2.17 MPa where the p-value <0.000 showed significant difference in bond strength between B1 and B2.
Mean bond strength for groups B1 & B2
| Group | Mean (MPa | Standard deviation |
|---|
| Group B1 | 14.91 | 2.99 |
| Group B2 | 19.92 | 2.17 |
T-test for equality of means for groups B1 & B2
| Variances | Levene’s test for equality of variances | t-test for equality of means |
|---|
| F | Sig. | T | df | Sig.(2-tailed) | Mean difference |
|---|
| Equal variances assumed | 0.51 | 0.48 | -4.28 | 18 | .000 | -5.01 |
| Equal variances not assumed | | | -4.28 | 16.41 | .001 | -5.01 |
[Table/Fig-10,11] showed mean bond strength for groups A1 was 16.03±2.92 MPa and B1 14.91±2.99 MPa where the t-test for equality of means for groups A1 & B1 where the p value <0.40 showed no significant difference in bond strength between A1 and B1.
Mean bond strength for groups A1 & B1
| Group | Mean (MPa | Standard deviation |
|---|
| Group A1 | 16.03 | 2.92 |
| Group B1 | 14.91 | 2.99 |
T-test for equality of means for groups A1 & B1
| Variaences | Levene’s test for equality of variances | t-test for equality of means |
|---|
| F | Sig. | T | df | Sig.(2-tailed) | Mean difference |
|---|
| Equal variaences assumed | 0.07 | 0.78 | 0.847 | 18 | .408 | 1.12 |
| Equal variaences not assumed | | | 0.847 | 17.98 | .408 | 1.12 |
[Table/Fig-12,13] showed mean bond strength for groupA2 was 21.26±3.56 MPa and B2 19.92±2.17 MPa where the p-value <0.32 showed no significant difference in bond strength between A2 and B2.
Mean bond strength for groups A2 & B2
| Group | Mean (MPa | Standard deviation |
|---|
| Group A2 | 21.26 | 3.56 |
| Group B2 | 19.92 | 2.17 |
T-test for equality of means for groups A2 & B2
| Variaences | Levene’s test for equality of variances | t-test for equality of means |
|---|
| F | Sig. | T | df | Sig.(2-tailed) | Mean difference |
|---|
| Equal variaences assumed | 3.04 | 0.09 | 1.01 | 18 | 0.326 | 1.34 |
| Equal variaences not assumed | | | 1.01 | 14.87 | 0.326 | 1.34 |
[Table/Fig-14] showed the comparison of mean shear bond strength for four groups A1, A2, B1 & B2. Group A2 showed maximum shear bond strength coMPared to other groups.
Shear bond strength for groups A1, A2, B1 and B2
Discussion
Ceramics appearance can be customized to simulate the colour, translucency and fluorescence of natural teeth. Drawback of using ceramics as tooth replacement materials is their very low fracture toughness and fracture occurs at a very low strain of about 0.1% [24]. Acid etching and adhesive luting can greatly limit the process of crack growth through the glass matrix resulting in the ultimate failure of the restoration by a process of crack bridging at the bonded interface of the porcelain by luting composite [25].
CAD/CAM provides new options for dentistry, creating an alternative to the conventional impression and casting techniques for providing dental restorations. CAD/CAM system allows the fabrication of single and multiple unit frame works as well as implant components. Resin-based composites are the material of choice for the adhesive luting of ceramic restorations.
Resin-dentin adhesion of successful bonding systems occurs in 3 steps they are etching, priming and bonding resin application. Etching of dentin followed by water rinsing. Subsequently the hydrophilic primer is applied to increase dentin surface energy and to facilitate the penetration of the bonding resin monomer, generating a mixed zone of resin-entangled collagen fibrils, known as hybrid layer [26]. The quality of resin dentin adhesion can greatly be influenced by the duration of etching process, and by the amount of dentin surface humidity following rinsing of the acid and prior to resin infiltration.
One step self-etch or so called all-in-one adhesive was introduced combining the conditioning, priming, and bonding resin in a single step. Generally the formulation of the self-etching primers to combine with resin cements includes an aqueous mixture of acidic monomers, such as a phosphate ester or a carboxylic acid and hydrophilic monomers such as hydroxylethylmethacrylate. Due to their intrinsic acidity, these primers can simultaneously condition and prime the hard tooth tissues, using the smear layer as an intermediate bonding substrate.
In the present study two different types of bonding agents were used, first was an etch and rinse bonding agent (total etch bonding agent), and second was a self etching bonding agent. Self-etching adhesives use the smear layer as a bonding substrate. Self-etching primers are applied to the dentin surface covered with the smear layer with the objective of incorporating the smear layer into the hybrid layer. In these systems the dentinal tubules are not exposed due to a reduction in the number of bonding steps, resulting in reduce postoperative sensitivity [27].
Concerns surrounding the self-etching adhesives include the ability of the self-etching primer to penetrate a thick smear layer and the reduced potential for demineralization in the sub-surface dentin due to neutralization [28]. Systems can be classified into mild, moderate and aggressive. On the basis of acidity of the primer and its ability to dissolve and/or penetrate dentin smear layers [26].
Micromechanical interlocking and chemical bonding is required for strong resin bond to the ceramic surface, which requires roughening and cleaning for adequate surface activation. Acid etching with solutions of ammonium bifluoride or hydrofluoric acid can achieve proper surface texture and roughness [29]. The glassy matrix is selectively removed, and crystalline structures are exposed. Hydrofluoric solutions between 2.5% and 10% applied for 2 to 3 minutes seem to be most successful.
Two different surface treatments were used one is etching with < 5% hydrofluoric acid and second is etching with < 5% hydrofluoric acid and application of silane coupling agent. In the present study < 5% hydrofluoric acid was used to etch the ceramic surface. Etching selectively dissolve the glassy or crystalline components of the ceramic and produce a porous irregular surface, this would increase the surface area and facilitate the penetration of the resin in to the micro retentions of the etched ceramic surfaces, thus improving wettability.
Application of a silane coupling agent to the pretreated ceramic surface provides a chemical covalent and hydrogen bond and is a major factor for a sufficient resin bond to silica-based ceramic. Silanes are bifunctional molecules that bond silicone dioxide with the OH groups on the ceramic surface. They have a degradable functional group that copolymerizes with the organic matrix of the resin. A silane coupler and a weak acid are the constituents of a silane coupling agents. Wettability of the ceramic surface is increased by silanization [30].
In this study the shear bond strength for Prime & Bond NT ranges from 8.9 MPa to 19.2 MPa which were etched with < 5%hydrofluoric acid, whereas the bond strength for Xeno III ranges from 9.2MPa to 18.7 MPa, which were etched with < 5% hydrofluoric acid. The adhesion values obtained in this study are in accordance with values reported by other authors [29], despite a difficult comparison of results because of variables such as specimen preparation technique, crosshead speed, cross-sectional surface area, and the type of test.
Limitations of This Study
The samples were stored in water, which does not fully represent the dynamic environment of the oral cavity presented by saliva with short duration.
Clinical implication: Accurate and meticulous procedures during the cementation phase may play an essential clinical role in achieving a valuable connection between the dentin and the ceramic restoration. Etching with hydrofluoric acid followed by the application of silane coupling agents, have been able to increase the adhesion strength to the feldspathic ceramic.
Conclusion
Within the limitations of this study, the following conclusions were obtained. The shear bond strength between CAD/CAM ceramic and dentin with Prime & Bond NT and < 5%hydrofluoric acid and silane coupling agent as surface treatments (21.26±3.561 MPa) was more than the other surface treatment methods.