Pleomorphic Adenoma of Cheek Masquerading as Fibrolipoma – Case Report with Review
Nagalaxmi Velpula1, Swetha Reddy Annam2, Supriya Reddy Pallepati3, Revanth Kumar4, Ashwanth Kumar5
1 Professor and Head of the Department, Department of Oral Medicine and Radiology, Sri Sai College of Dental Surgery, Vikarabad, Ranga Reddy District, Telangana, India.
2 Post Graduate, Department of Oral Medicine and Radiology, Sri Sai College of Dental Surgery, Vikarabad, Ranga Reddy District, Telangana, India.
3 Post Graduate, Department of Oral Medicine and Radiology, Sri Sai College of Dental Surgery, Vikarabad, Ranga Reddy District, Telangana, India.
4 Post Graduate, Department of Oral and Maxillofacial Surgery, Sri Sai College of Dental Surgery, Vikarabad, Ranga Reddy District, Telangana, India.
5 Post Graduate, Department of Oral and Maxillofacial Surgery, Sri Sai College of Dental Surgery, Vikarabad, Ranga Reddy District, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Swetha Reddy Annam, H. NO- 3-65/10, Mubarak Nagar, Nizamabad- 503003, India. E-mail : swethareddy.bds@gmail.com
Salivary gland tumours are rare, comprising 3% of head and neck tumours, of which Pleomorphic Adenoma (PA) constitutes 70-80%. It accounts for 53-74% of parotid tumours, 44-68% submandibular gland tumours and 38-43% of minor salivary gland tumours. It usually presents as painless, firm, slow growing mobile mass. Various diagnostic modalities for early detection of the tumour include FNAC, Ultrasonography, CT and MRI. The choice of treatment of pleomorphic adenoma of the minor salivary glands depends on the aggressiveness of the tumour, the extension of the mass, and its relation with the vital structures. Here, we report a rare case of pleomorphic adenoma of minor salivary glands of cheek in a 40-year-old male patient with emphasis on various diagnostic modalities.
Benign neoplasm, Minor salivary gland, Parotid gland, Ultrasound
Case Report
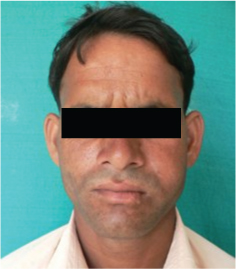
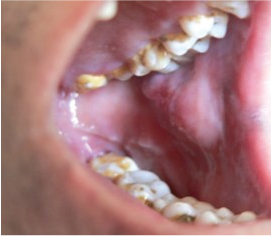
A 40-year-old male patient reported to the outpatient Department of Oral Medicine, Diagnosis and Radiology of Sri Sai College of Dental Surgery, Vikarabad, Telangana with a chief complaint of pain and swelling on left side of face since one year which was gradually increasing in size. Extra-orally, a swelling was seen on the middle 3rd of left side of face measuring approximately of size 2x2 cm [Table/Fig-1], it was mildly tender, freely movable over the underlying structures, firm in consistency. Intraorally, a swelling was seen on left buccal mucosa approximately of size 2x2 cm along the level of occlusion, consistency was firm and mildly tender on palpation [Table/Fig-2]. Based upon the clinical findings, a provisional diagnosis of “FIBROLIPOMA” was given.
Swelling on left side of cheek, extraorally,
Swelling on left buccal mucosa, intraorally,
Investigations
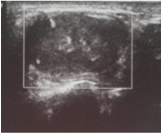
Orthopantomograph was advised to rule out any presence of calcifications; however, which did not show any evidence of such calcifications in the lesion. FNAC report revealed variably cellular aspirate, showing single and poorly cohesive clusters of epithelial cells with fibrillar, fibro myxoid stroma suggestive of pleomorphic adenoma. Ultrasonography showed evidence of a well defined mildly heterogenous hypoechoic lesion measuring 2.4x1.5 cm with few tiny calcifications within [Table/Fig-3].
Ultrasonography showing heterogenous hypoechoic lesion
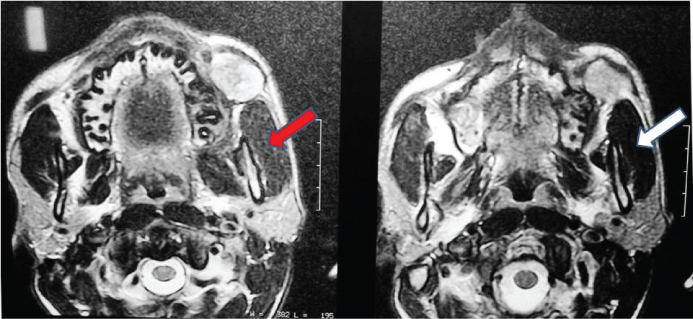
MRI showed evidence of well defined T2 heterogenous hyperintense lesion displacing left buccinator muscle anteriorly. Tiny T1 hyper intense, T2 hypointensity seen in periphery of lesion posteriorly – significant of calcification [Table/Fig-4].
Axial section of MRI showing T2 heterogenous hyperintense lesion displacing left buccinators muscle anteriorly (in red arrows). Tiny T1 hyperintense, T2 hypointensity seen in periphery of lesion posteriorly (in white arrows)
Routine blood investigations (Hb, RBC, Differential leukocyte count etc) were done, as listed in [Table/Fig-5]. The patient was posted for surgery after obtaining an informed consent.
Investigations and surgical profile of the patient
| Investigations |
|---|
| Hemoglobin | 14.5gm % |
| Erythrocyte count | 4.8 million per cu mm |
| Leucocytes | 9800 per cu mm |
| Eosinophils | 02% |
| Basophils | 0 |
| Neutrophils | 65% |
| Lymphocytes | 31% |
| Monocytes | 2% |
| Creatinine | 0.6 mg / dl |
| Blood urea | 21 mg / dl |
| Blood grouping | A+ve |
| HIV | Non reactive |
| HBS Ag | Non reactive |
| Random blood sugar level | 90 mg / dl |
| Bleeding time | 2 min 30 sec |
| Clotting time | 6 min 10 sec |
Surgical technique
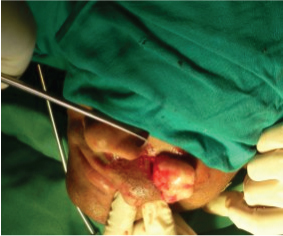
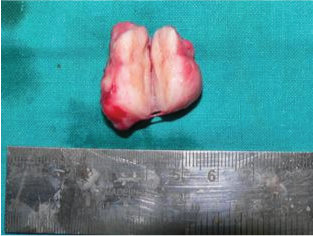
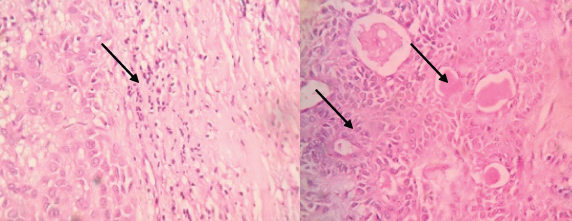
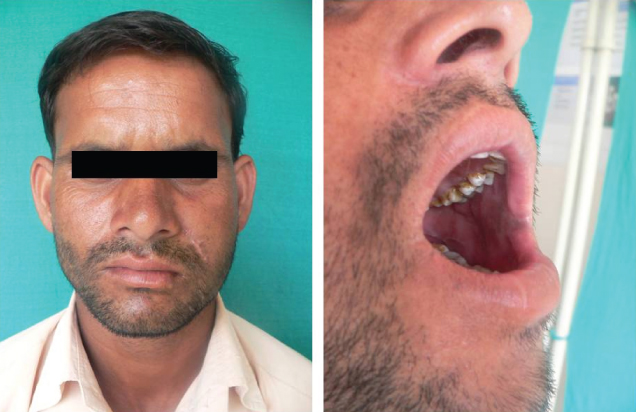
Surgical intervention planning was done taking into account the fact that the lesion appeared benign and was superficial and mobile. Under strict aseptic conditions the patient was operated under local anaesthesia for wide local excision of the mass and primary closure of adjacent mucosa. Extraoral incision was given on the prominent part of the swelling incising the skin, subcutaneous tissue and muscle. The tumour was present just below the cheek skin near the parotid gland and easily resectable. Extra oral incision was preferred because of the relative access to the underlying lesion. The tumour mass popped out as soon as the muscle layer was incised. The excised mass was 4x3 cm which was firm, rubbery in consistency [Table/Fig-6,7]. Following excision of tumour, surgical wound was closed in layers in a tension-free water tight fashion. The excised mass was sent for histopathological examination. Presence of combination of glandular epithelium and mesenchymal tissue with changes like accumulation of mucoid material, foci of hyalinization and the epithelial component composed of myoepithelial cells arranged in cords and nests confirmed it as case of a pleomorphic adenoma on histopathological examination [Table/Fig-8]. Postoperative follow up of 6 months showed no recurrence [Table/Fig-9].
Intraoperative photograph,
Excised surgical specimen
Histopathology: The Haematoxylin & Eosin stained histopathological sections showed combinations of glandular epithelium and mesenchymal tissue with changes like accumulation of mucoid material, foci of hyalinization showed in arrows. The epithelial component is made of myoepithelial cells arranged in cords and nests
Postoperative Photograph after 6 months with no evidence of recurrence or any pathology
Discussion
Pleomporphic adenoma (PA) is recognised as the most common benign salivary gland tumour [1]. The term Pleomporphic adenoma was coined by Willis [2]. PA is defined by WHO in 1972 as “a circumscribed tumour characterized by its pleomorphic or mixed appearance clearly recognizable epithelial tissue being intermingled with tissue of mucoid, myxoid or chondroid appearance” [3]. It occurs in 3rd to 6th decade of life with slight female predilection, with female to male ratio of 2:1 [4]. Although, it constitutes a mixture of epithelial and mesenchymal cells [5], the term “mixed tumour” is a misnomer [2]. The parotid gland is commonly involved of which lower pole of the superficial lobe is a more common site. An 8% of PAs involve the minor salivary glands of which 60-65% involves the palate [2] and 5.5% the minor salivary glands of cheek [6].
Immunohistochemical expression of mucins MUC 1, MUC 2, MUC 4, MUC 5AC and MUC 6 was evaluated by Hamada et al., [7]. They observed increased expression of MUC1/DF3 in recurrent cases. It presents as a painless, slow growing, firm, mobile mass, irregular in shape [2,5,8] ulceration is rarely seen [2,8] grows between ascending ramus and stylomandibular ligament, resulting in dumb-bell shaped tumour that is misdiagnosed as a tumour of lateral pharyngeal wall or soft palate [5]. They do not invade the underlying structures owing to their benign nature [2]. The malignant transformation rate is 8.5% [8]. Carcinoma ex pleomorphic adenoma and metastatizing benign mixed tumour are the two variants that undergo malignant transformation [9]. PA of cheek should be differentiated from lipoma, neurofibroma, buccal abscess, rhabdomyoma, sebaceous cyst and mucoepidermoid carcinoma [10]. The important diagnostic modalities are Fine Needle Aspiration Cytology (FNAC) and imaging modalities which include Ultrasonography, Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). FNAC, a widely accepted preoperative diagnostic tool as the diagnostic accuracy with it is 80-95% [11]. Ultrasonography can be used for the detection of size, location, presence/ absence of calcifications, and also to estimate extraglandular/ intraglandular position [12]. CT shows the extent of bony invasion. CT may show irregular borders which may be misdiagnosed for malignancy; MRI stands superior in delineating the capsule and shows better soft tissue resolution [13]. Axial section of MRI in the present case showed T2 heterogenous hyperintense lesion displacing left buccinators muscle anteriorly and a tiny T1 hyperintense, T2 hypointensity seen in periphery of lesion posteriorly.
Histopathologically, PA has incomplete fibrous capsule or are unencapsulated. The epithelial components forms ducts, small cysts, small cellular nests, sheets of cells, anastomosing cords and foci of keratinizing squamous or spindle cells. Myoepithelial cells appear as angular or spindled, while some cells are round with eccentric nuclei and hyalinized eosinophilic cytoplasm. Extensive accumulation of mucoid material gives rise to mucoid appearance [2]. The cut sections under histopathological examination showed combinations of glandular epithelium and mesenchymal tissue with changes like accumulation of mucoid material, foci of hyalinization showed in arrows. The epithelial component is made of myoepithelial cells arranged in cords and nests along with presence of normal salivary architecture in form of acini.
Surgical excision (wide local tumour resection with safety margins particularly in present case of cheek) is the treatment of choice for PA. For the lesions of superficial parotid lobe, superficial parotidectomy with preservation of facial nerve is performed. If deep lobe is involved, total parotidectomy with preservation of facial nerve should be performed. Prognosis is excellent with a cure rate of more than 95% [5]. Recurrence may be due to conservative or inadequate enucleation or rupture of capsule [14] or due to spillage of tumour cells. After the treatment, the patients should be under regular follow ups. These tumours are radio resistant [2]; Radiation therapy can be an adjuvant to surgery to decrease further recurrence rates however it carries a minute but definite risk of malignant transformation of 3-4% [15,16].
Conclusion
Pleomorphic adenoma of minor salivary gland especially involving cheek is a relatively rare lesion and poses challenge to the most experienced oral physician. It should be considered in differential diagnosis to rule out swellings of the cheek. Surgical excision of the tumour with wide margins is the treatment of choice. So early diagnosis, prompt treatment and regular follow up and close monitoring at least for 5 years is necessary to prevent the risk of recurrence and rare chances of malignant transformation.
[1]. Ito FA, Jorge J, Vargas PA, Histopathological findings of pleomorphic adenomas of the salivary glandsMed Oral Patol Oral Cir Bucal 2009 14(2):57-61. [Google Scholar]
[2]. Rajendran S, Sivapathasundaram S, Shafer’s Textbook of Oral Pathology 2009 6th editionElsevier:219-24. [Google Scholar]
[3]. Verma P, Sachdeva SK, Verma KG, Pleomorphic adenoma of cheek: A rare case report and review of literatureIndian J Dent Res 2014 25:122-24. [Google Scholar]
[4]. Rivera-Bastidas H, Ocanto RA, Acevedo AM, Intraoral minor salivary gland tumours a retrospective study of 62 cases in a Venezuelan populationJ Oral Pathol Med 1996 25:1-4. [Google Scholar]
[5]. Neville BW, Damm DD, Allen CM, Oral & Maxillofacial Pathology 2009 3rd edSt. LouisSaunders Elsevier:477-79. [Google Scholar]
[6]. Toida M, Shimokawa K, Makita H, Intraoral minor salivary gland tumours: a clinicopathological study of 82 casesInt J Oral Maxillofac Surg 2005 34:528-32. [Google Scholar]
[7]. Hamada T, Matsukita S, Goto M, Mucin expression in pleomorphic adenoma of salivary gland: a potential role for MUC1 as a marker to predict recurrenceJ Clin Pathol 2004 57:813-21. [Google Scholar]
[8]. Greenberg MS, Glick M, Ship JA, Burkett’s textbook of Oral medicine 2008 11th edBC Becker Inc:217-18. [Google Scholar]
[9]. Sunil S, Gopakumar D, Pleomorphic adenoma. A case report and review of literatureInt J Odontostomat 2013 7(2):171-74. [Google Scholar]
[10]. Jorge J, Pires FR, Alves FA, Juvenile intraoral pleomorphic adenoma: Report of five cases and review of the literatureInt J Oral Maxillofac Surg 2002 31:273-75. [Google Scholar]
[11]. Kapadia SB, Dusen Berry D, Dekker A, Fine Needle Aspiration of Pleomorphic adenoma and Adenoid cystic carcinoma of Salivary gland originActa Cytol 1997 41:487-92. [Google Scholar]
[12]. Bialek EJ, Jakubowski W, Karpinska G, Role of ultrasonography in diagnosis and differentiation of p leomorphic adenomaArch Otolaryngol Head Neck Surg 2003 129:929-33. [Google Scholar]
[13]. Melkundi M, Babaji P, Saikhedkar R, Pleomorphic adenoma of parotid gland: a case reportOral & Maxillofacial Pathology Journal 2012 3:228-31. [Google Scholar]
[14]. Dalati T, Hussein MR, Juvenile pleomorphic adenoma of the cheek: a case report and review of literatureDiagnostic Pathology 2009 4:32 [Google Scholar]
[15]. Friedrich RE, Li L, Knop J, Pleomorphic Adenoma of the Salivary Glands: Analysis of 94 PatientsAnticancer research 2005 25:1703-06. [Google Scholar]
[16]. Mendenhall WM, Mendenhall CM, Werning JW, Salivary gland pleomorphic adenoma review articleAmerican Journal of Clinical Oncology 2008 31(1):95-99. [Google Scholar]