Cystic Change in Pleomorphic Adenoma: A Rare Finding and a Diagnostic Dilemma
Shaan Khetrapal1, Sujata Jetley2, Mohd. Jaseem Hassan3, Zeeba Jairajpuri4
1 Demonstrator, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi, India.
2 Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi, India.
3 Assistant Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi, India.
4 Associate Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Zeeba Jairajpuri, Associate Professor, Department of Pathology, Hamdard Institute of Medical Sciences and Research, Hamdard Nagar, New Delhi 110062, India.
E-mail: jairajpurizs@gmail.com
Pleomorphic adenoma forms the majority of salivary gland neoplasms. Cystic change in pleomorphic adenomas is a diagnostic dilemma and can mimic mucoepidermoid carcinoma, mucocele or carcinoma ex pleomorphic adenoma and squamous cell carcinoma. Hereby we report this interesting and rare case of cystic pleomorphic adenoma in a 32-year-old male.
Histological diversity, Parotidectomy, Salivary gland neoplasms
Case Report
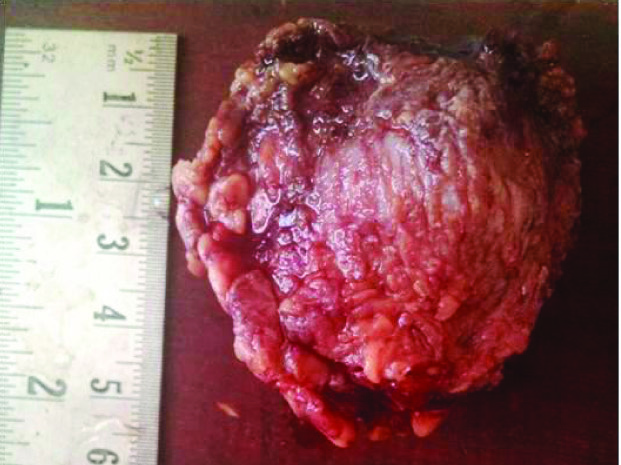
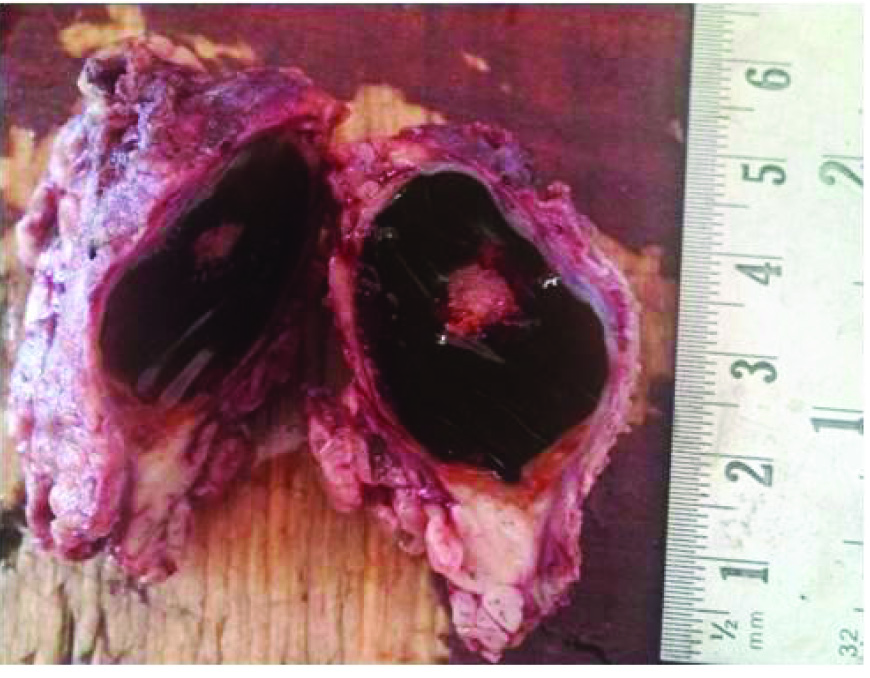
A 32-year-old male presented with a swelling on left side of the face, parotid as well as posterior auricular region of 4 months duration. On local examination a 3 X 2 cm firm to hard, fluctuant swelling was seen. It was non tender with normal overlying skin. On ultrasonography, a complex cystic lesion in left parotid region was reported. On fine needle aspiration cytology (FNAC), the smears showed numerous cystic macrophages against a background of fluid and squamoid metaplastic cells intermixed with bland epithelial cells and was reported as a cystic lesion of parotid gland. Subsequently a superficial parotidectomy was performed. On gross examination a 5×5×3 cm grey brown colour, irregular soft tissue was received [Table/Fig-1]. On cut section it revealed a single cystic cavity having smooth surface filled with gelatinous material [Table/Fig-2]. Histopathology revealed a cystic lumen partially filled with thick mucinous material lined by low cuboidal and metaplastic epithelial cells [Table/Fig-3]. The wall of the cavity showed areas of chronic inflammatory infiltrate, haemosiderin laden macrophages and cholesterol clefts. A biphasic population of epithelial and mesenchymal cells was seen. Epithelial cells were glandular having large hyperchromatic nuclei and the stromal component comprised of myxoid as well as ill-defined chondroid areas [Table/Fig-4]. Adjacent to the lesion, unremarkable salivary gland tissue was identified. The lesion was diagnosed as a pleomorphic adenoma with cystic change. Subsequent to the surgery the lesion has healed uneventfully.
A grey brown colour, irregular, intact soft tissue
Cut section showing a single cystic cavity having smooth surface filled with gelatinous material
Section shows a cystic lumen filled with thick mucinous material, the wall of the cavity showing epithelial component along with an area of areas of unremarkable, salivary gland tissue

(40X) Section shows epithelial as well as stromal component comprising of ill defined chondro myxoid areas

Discussion
Pleomorphic adenoma (PA) forms the largest group of salivary gland neoplasm, constituting approximately 64% [1]. It is a mixed tumour characteristically showing a combination of epithelial and mesenchymal elements [2]. This histological diversity although being the hallmark of PA can at times pose difficulty in correct interpretation [3,4]. Cystic change in pleomorphic adenomas is a diagnostic dilemma and can mimic mucoepidermoid carcinoma, mucocele or carcinoma ex pleomorphic adenoma and squamous cell carcinoma [5].
Pleomorphic adenoma characteristically shows variable morphological patterns, architecture and cellularity [5]. It can present in a plethora of findings ranging from the varying proportions between epithelial and chondromyxoid stroma, varying histological patterns among different parts of tumours, metaplastic variation and cystic changes [3]. FNAC is a valuable tool in the evaluation of salivary gland masses, avoiding surgery for many patients and in others allowing formulation of a preoperative plan that encompasses a limited or radical surgical approach [1]. The appearance of the aspirated material of pleomorphic adenoma is characteristic, showing droplets of thick or pasty mucoid material. Cellularity is variable, but majority of the cases are cellular composed of extracellular matrix, myoepithelial and ductal cells in various proportions and stroma [6]. However, in our case the cytology yield was scant.
Although the diagnosis of PA is usually made with ease on cytology, cystic PAs pose certain diagnostic challenges. PA, representing a large number of salivary gland neoplasms, is usually easily recognized when evaluated by FNAC, however metaplastic changes, degeneration and altered differentiation can pose a diagnostic challenge [1]. This has been emphasized by authors that there is need for a cautious and systematic approach in the cytologic and histopathologic interpretation of cystic pleomorphic adenoma with metaplastic epithelial changes [7]. Considerable variation in the proportions of the constituent elements is a challenge resuting in differential which include low-grade carcinomas, monomorphic adenoma, metastases, and the plasmacytoid appearance of the myoepithelial cells may be mistaken for malignant lymphoma or plasma cell proliferations [6]. However, as seen in the present case FNAC was non contributory and histopathological evaluation had to be done as morphological diversities are often not a problem in histopathology, where the whole tumour is available for examination.
Histopathology of the present case revealed focal metaplastic change. Squamous metaplastic changes seen in benign salivary glands often lead to diagnostic difficulties or misdiagnosis, simulating malignant neoplasm such as mucoepidermoid carcinoma and squamous cell carcinoma. Possibly, due to nuclear atypia in squamous cells and scanty mesenchymal component in pleomorphic adenoma. [8]
Low-grade mucoepidermoid carcinoma may be one of the most difficult salivary gland neoplasms to exclude in cytologic preparations due to its cystic nature and often difficult to recognize bland mucin-containing cells. Characteristic metachromatic fibrillar chondromyxoid stroma, which usually is seen in PAs, is important for diagnosis of PA. It was not seen in the present aspirate similar to other authors [2]. Although cytodiagnosis of PAs is suggested based on the presence of other cellular components, resection is recommended. Smears should be closely scrutinised for fragments of chondromyxoid stroma to avoid misinterpretation of pleomorphic adenoma in the presence of squamous metaplasia as a mucoepidermoid carcinoma on cytology [9]. Presence of squamous, mucinous, and/or sebaceous metaplasia, especially in the absence of chondromyxoid stroma, presents the potential for misinterpretation of the FNAB as indicative of malignancy in general and MEC in particular [2]. The subsequent parotidectomy usually reveals an encapsulated cystic PA with mixed appendageal differentiation. Chondromyxoid stroma is only focally present. Histopathological evaluation becomes necessary in such cases where cytology has not been helpful. This becomes essential when low grade mucepidermoid carcinoma is a prominent differential diagnosis and needs to be excluded. Diagnostic difficulty in interpretation of cytological material often arises due to sparse cellularity, lack of observer familiarity with the different patterns and limited selective sampling. In the absence of chondromyxoid stroma, squamous metaplasia, basaloid cells and hyaline globules may be misinterpreted as carcinoma [9].
On review of literature to the best of our knowledge only a few cases of PA with cystic change have been reported [3,5,10–12]. Cyst formation might originate from the squamous metaplasia of tumour cells, enlargement of duct-like structures by secretion from tumour cells or salivary gland tissue, haemorrhagic infraction and necrosis in the tumour [13]. Squamous metaplasia is uncommon and incidental microscopic finding in benign salivary gland tumours. This change is commonly associated with repair following infarction and necrosis of the salivary glands. The metaplastic process might occur spontaneously or be triggered by minor trauma. The most probable etiology for this change is ischemia [8]. The lining epithelium is often flattened and denuded at places. Cystic changes in pleomorphic adenoma of minor salivary glands are rare and they might be accompanied by cellular changes suggestive of malignancy, however it was not seen in this particular case where no cellular atypia or mitosis was observed [5]. Extensive squamous metaplasia with cystic changes in PA, especially in scanty or absent stroma, can lead to a misdiagnosis of benign condition like keratocystoma or malignancy including mucoepidermoid carcinoma and squamous cell carcinoma, due to limited and selective sampling by FNAC and incisional biopsy [13,14]. Florid squamous metaplasia as well as adenexal differentiation in the form of keratin filled cysts has been reported in literature [9]. To avoid misinterpretation of PA with squamous metaplasia as squamous cell carcinoma or MEC on FNAC, a close scrutiny for chondromyxoid stroma, a characteristic feature of pleomorphic adenoma, is important.
Conclusion
Cystic change in pleomorphic adenoma is a rare finding and a diagnostic challenge. Although, FNAC is a good preoperative procedure for the diagnosis of PA. One should be aware of the cytological variations to avoid diagnostic errors. A targeted adequate sampling is suggested to exclude the other cystic lesions in the differential diagnosis of salivary glands. In order to avoid unnecessary aggressive therapy, awareness of this rare benign possibility is essential so that misinterpretation of the pathological findings as malignant are avoided.
[1]. Brachtel EF, Pilch BZ, Khettry U, Zembowicz A, Faquin WC, Fine-Needle Aspiration Biopsy of a Cystic Pleomorphic Adenoma with Extensive Adnexa-Like Differentiation: Differential Diagnostic Pitfall with Mucoepidermoid CarcinomaDiagnostic Cytopathology 2002 28:100-03. [Google Scholar]
[2]. Siddiqui NH, Wu SJ, Fine-Needle Aspiration Biopsy of Cystic Pleomorphic Adenoma with Adnexa-Like Differentiation Mimicking Mucoepidermoid Carcinoma: A Case ReportDiagnostic Cytopathology 2005 32:229-32. [Google Scholar]
[3]. Lim S, Cho I, Park JH, Lim SC, Pleomorphic Adenoma with Exuberant Squamous Metaplasia and Keratin Cysts Mimicking Squamous Cell Carcinoma in Minor Salivary GlandOpen Journal of Pathology 2013 3:113-16. [Google Scholar]
[4]. Das DK, Anim JT, Pleomorphic adenoma of salivary gland: to what extent does fine needle aspiration cytology reflect histopathological features?Cytopathology 2005 16:65-70. [Google Scholar]
[5]. Sudheendra US, Shashidara R, Chakki AB, Sreeshyla HS, Cystic Pleomorphic Adenoma of Minor Salivary GlandsInternational Journal of Dental Clinics 2011 3(2):107-08. [Google Scholar]
[6]. Mukunyadzi P, Review of Fine Needle Aspiration cytology of salivary gland neoplasms, with emphasis on differential diagnosisAm J Clin Pathol 2002 118:100-15. [Google Scholar]
[7]. Siddaraju N, Murugan P, Basu D, Verma SK, Preoperative cytodiagnosis of cystic Pleomorphic adenoma with squamous metaplasia and cholesterol crystals: a case reportActa Cytol 2009 53:101-04. [Google Scholar]
[8]. Kaveri H, Gopalkrishnan K, Venkatesh A, Cystic and florid Squamous metaplasia in pleomorphic adenoma of palate- A Diagnostic DilemmaAsian J of Med Sc 2014 5:108-10. [Google Scholar]
[9]. Ramraje SN, Sisodia SM, Goel A, Fine-Needle Aspiration Cytology Of Pleomorphic Adenoma: Cytologic Variations And Diagnostic Pitfalls: A Report Of Two CasesNJIRM 2014 5:133-37. [Google Scholar]
[10]. Chen Y, Lin C, Lai S, Chen C, Wang W, Lin Y, Pleomorphic adenoma with extensive necrosis: report of two casesOral Diseases 2004 10(1):54-9. [Google Scholar]
[11]. Maji J, Soniya A, Jagadish C, Pleomorphic adenoma with extensive cystic degeneration: A rare presentationIndian Journal of Stomatology 2011 2:123 [Google Scholar]
[12]. Ellis GL, Auclair PL, Atlas of tumour pathology: tumours of the salivary glandsWashington: Armed Forces Institute of Pathology1996:39-153. [Google Scholar]
[13]. Nasit JG, Dhruva G, Extensive squamous metaplasia with cystic change in pleomorphic adenoma: A potential diagnostic pitfall in fine needle aspiration cytologyClin Cancer Investig J 2013 2:166-69. [Google Scholar]
[14]. Lam KY, Ng IO, Chan GS, Palatal pleomorphic adenoma with florid squamous metaplasia: A potential diagnostic pitfallJ Oral Pathol Med 1998 27:407-10. [Google Scholar]