Thyroid carcinoma is the most common endocrine malignancy and more than 95% of thyroid carcinoma originates from follicular epithelial cells [1]. Incidence of thyroid carcinomas derived from follicular cells varies in different parts of the world, but most countries have reported increased incidence during the past few decades.
Most follicular cell derived carcinomas are well differentiated malignancies which can be treated effectively by surgical resection [2]. Preoperative evaluation and establishing a diagnosis of apparently single thyroid nodule by fine needle aspiration cytology is challenging [3]. In many cases especially in follicular-patterned thyroid lesions, even with histological analysis the diagnosis of neoplasm and distinction between benign and malignant neoplasm can be difficult [2,4,5]. Exclusive follicular pattern in adenomatoid nodules and disruption of capsule in adenomas create difficulties in diagnosis by histopathology [2]. However, papillary carcinomas are also prone to diagnostic discrepancies among pathologists [5].
The decision favouring benign or malignant lesion has clinical consequence and implies different modalities of further treatment and management. In this regard, the diagnostic approach to these tumours should include IHC markers that can aid in better assessment of morphologic details [2]. Many IHC markers including Gal-3 have been evaluated in this regard. Galectins are a family of lectins that are predominantly localized in the cytoplasm, may translocate to the perinuclear membrane, nucleus and/or get secreted from the cytoplasm [3,6,7]. Gal-3 possesses affinity for beta galactoside containing intracellular, extracellular and cell surface associated glycoconjugates and thus participates in cellular functions like cell proliferation, apoptosis, cellular transformation, tumour progression and metastasis of cancer cells [6–8]. In the recent times Gal-3 has received notable recognition for its usefulness as a diagnostic marker for thyroid cancer [8]; the utility assessed by studies [5,9,10] have reported encouraging results.
In the present study the differential expression of Gal-3 in benign and malignant thyroid neoplasms is evaluated in terms of intensity and the proportion of tumour cells stained with the immunomarker.
Materials and Methods
We examined specimens taken from 50 consecutive patients who were operated for either benign or malignant thyroid disease and accessioned at our institute from 2012 to 2014. Permission from the Institutional Ethics Committee for the study was obtained. In the pathology department all these surgical specimens were fixed in 10% buffered formalin, routinely processed, paraffin-embedded and the sections were stained with haematoxylin & eosin stain. The specimens included lobectomy, isthmectomy, hemithyroidectomy, subtotal, near total and total thyroidectomy with macroscopically and microscopically detected neoplastic lesion. The histopathological evaluations were performed by two pathologists and the final diagnosis of the tumour histotype and subhistotype was made in accordance with World Health Organization classification [11]. All the cases had unambiguous histomorphology and there was 100% agreement between the two pathologists. For statistical purposes the histopathologic diagnosis was considered the gold standard. The diagnoses of the neoplastic lesions were distributed as follows: 23 PTC, 2 FTC, 2 MTC, 1 insular carcinoma, 1 ATC, 1 metastatic adenocarcinoma, 17 FA, 2 Hurthle cell adenoma and 1 hyalinizing trabecular adenoma cases.
Immunohistochemistry: IHC evaluation of the marker Gal-3 was performed on representative histologic sections of the thyroid neoplasms. Novolink polymer detection system was used. Sections were deparaffinised in xylene and rehydrated through ethanol. Antigen retrieval was done with Tri-sodium citrate buffer (pH 6.0 to 6.2) using Biogenex EZR microwave oven. Slides were brought to room temperature. Peroxidase and power blocks were done. After this primary antibody i.e., lyophilized mouse monoclonal Gal-3 antibody (Novacastra), dilution 1:100 was applied for an hour and washed with tris- buffered saline (TBS). Then super enhancer was added, for 20 minutes. Subsequently secondary antibody i.e. polymer horseradish peroxidase (HRP) was applied for 30 minutes, followed by washing with TBS. Then Diamine Benzidine (DAB) chromogen was applied for 5 minutes. Counter staining was done with Mayer’s Haematoxylin followed by clearing and mounting. Negative control sections were made by exclusion of the primary Gal-3 antibody. Positive control sections were obtained from sections of normal prostatic tissue.
Immunohistochemical Scoring
Distribution and intensity of Gal-3 cytoplasmic signal interpretation was based on the guidelines followed by Weber KB et al., [5] and Hermann ME et al., [10]. The staining intensity was graded on a scale of 0 to 3 where 0, 1+, 2+, and 3+ denote no staining, weak/slight staining, moderate staining and intense staining respectively, and the proportion of stained cells were interpreted as 1+ (< 5% of cells), 2+ (5% to 50% of cells) and 3+ (>50% of cells) [5,10]. As per the guidelines [5], cases that showed specific cytoplasmic staining of more than 5% of the tumour cells, regardless of the intensity, were scored as positive for Gal-3. The immunostained slides were scored by two pathologists and there was 100% concordance.
Sensitivity, specificity, predictive value, and diagnostic accuracy were assessed. Sensitivity was defined on the basis of thyroid cancer immunodetection as, the number of carcinomas with positive results as a percentage of the total number of thyroid cancers. Specificity was defined as, the number of benign thyroid lesions with negative results as a percentage of the total number of benign lesions. The positive and negative predictive values were respectively calculated as, the number of carcinomas with positive results as a percentage of the total number of cases with positive results, and the number of benign lesions with negative results as a percentage of total number of cases with negative results. Statistical significance of Gal-3 expression in various lesions was compared and analysed using 2x2 contingency table. Chi -square test with Yates correction was used to calculate P-value to ascertain statistical significance. p<0.05 was considered statistically significant.
Results
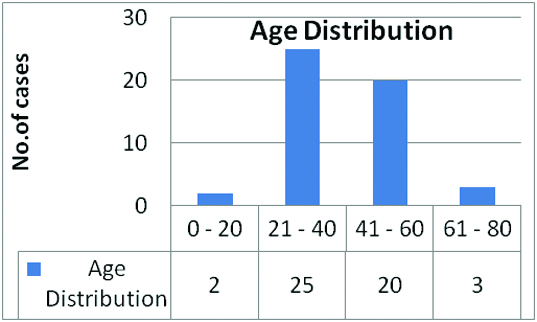
In the present study, 90% of the cases were in the age group of 20-60 years [Table/Fig-1]. Malignant neoplasms were more common (60%, 30 cases) than the benign neoplasms (40%, 20 cases) [Table/Fig-2,3]. Females constituted 72% of the cases. Out of the 23 cases of PTC, there were eight male and 15 female patients with a male to female ratio of 1:1.9. Of the remaining 7 cases of malignant neoplasms, all the cases of FTC, MTC, insular carcinoma and ATC were females, the single case of metastatic adenocarcinoma was a male patient. Among the 17 cases of FA, the male to female ratio was 1:3.3 with 4 males and 13 female patients. Both, Hurthle cell adenoma and hyalinizing trabecular adenoma patients were females.
Age distribution of all cases
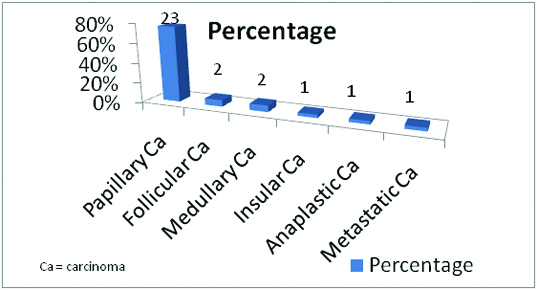
Distribution of histopathologic diagnoses in malignant neoplasms
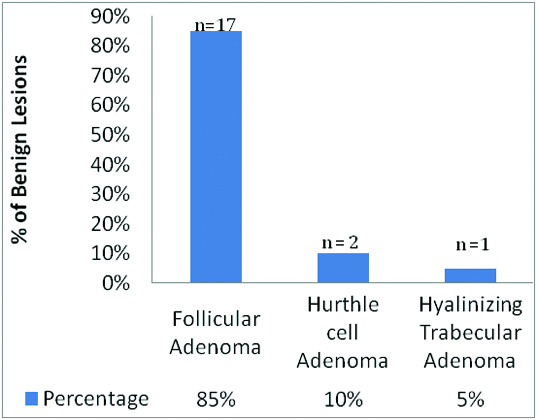
Distribution of histopathologic diagnoses of benign lesions
All the neoplasms were analysed for Gal-3 immunostaining [Table/Fig-4a-t]. Gal-3 stained the majority of malignant cases 26/30 cases (87%) in comparison to benign neoplasms, 3/20 cases (15%) and the difference was statistically significant (p-value <0.0001). Gal-3 expression in thyroid neoplasms was found to have a sensitivity of 86.67%, specificity of 85%, positive predictive value of 89.66% and negative predictive value of 80.95%. Gal-3 staining reactivity was seen mainly in the cytoplasm. In some cases of PTC, additional nuclear staining was also identified. However, no correlation between the location of reactivity as to nuclear and /or cytoplasmic and histological subtype was noted. The intensity grade and proportion score together with the Gal-3 expression interpretation in the 50 cases of thyroid neoplasms were documented. [Table/Fig-5].
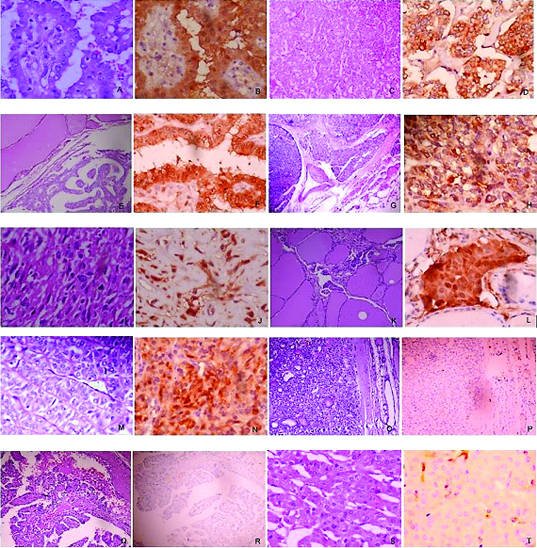
a&b) PTC: (a) H&E stain, 400X; (b) IHC Gal -3, 400X. Tumour cells showing intense staining (3+)
c&d) FVPTC: c) H&E, 100X; d) IHC Gal -3,400X; tumour cells showing intense cytoplasmic staining (3+)
e & f) Papillary microcarcinoma: e) H&E,100X; f) IHC Gal -3,400X; tumour cells showing intense cytoplasmic staining (3+)
g&h) FTC: g) H&E, 40X; with capsular infiltration; h) IHC Gal-3,400X; tumour cells showing intense cytoplasmic staining (3+)
i&j) ATC: i) H&E, 400X; scattered tumour cells; j) IHC Gal-3, 400X; tumour cells showing intense staining (3+)
k & l) Metastatic carcinoma: k) H&E, 100X; l) IHC Gal-3,400x; tumour cells showing intense staining (3+)
m&n) Hyalinizing trabecular adenoma: m) H&E, 400X; n) IHC Gal-3, 400X; tumour cells showing intense staining (3+)
o&p) Follicular adenoma: o) H&E, 100X; p) IHC Gal-3,100X; tumour cells showing no staining
q&r) PTC, classic type: q) H&E,100X; r) IHC Gal-3,100X; tumour cells showing no staining
s&t) Hurthle cell adenoma: s) H&E, 400X; t) IHC Gal-3, 400X; tumour cells showing no staining
Results of Galectin-3 Immunohistochemical staining
| Histologic diagnosis | Number of cases | Staining | Interpretation |
|---|
| I grade | P score |
|---|
| PTC: Classicn=14 | 110201 | 3+2+0 | 3+2+0 | PositivePositiveNegative |
| FVPTCn=4 | 010201 | 3+2+0 | 3+2+0 | PositivePositiveNegative |
| Micro carcinoma n=5 | 05 | 3+ | 3+ | Positive |
| FTC:n=2 | 0101 | 3+0 | 3+0 | PositiveNegative |
| MTC:n=2 | 0101 | 2+0 | 3+0 | PositiveNegative |
| Insular carcinoma | 01 | 2+ | 3+ | Positive |
| ATC | 01 | 3+ | 2+ | Positive |
| Metastatic carcinoma | 01 | 3+ | 3+ | Positive |
| Follicular Adenoman=17 | 120401 | 01+2+ | 01+2+ | NegativeNegativePositive |
| Hyalinizing Trabecular Adenoma | 01 | 3+ | 3+ | Positive |
| Hurthle cell Adenoman=2 | 0101 | 02+ | 02+ | NegativePositive |
I Score: Intensity score graded as 0 (no staining),1+ (slight staining), 2+ (moderate staining), or 3+ (intense staining)
P Score: Proportion of stained cells scored as 1+ (<5%of cells), 2+ (5%-50% of cells), or 3+ (>50% of cells)
Positive result= specific staining of more than 5% of the tumour cells with slight, moderate or intense staining
The predominant malignant neoplasm was PTC (23 cases) including 14 classic type, 4 follicular variant of PTC (FVPTC) and 5 microcarcinoma. 21 cases (91.3%) of PTC showed positive staining, 2 cases (8.7%) showed no staining of which one case was FVPTC and the other was classic type. Of the two cases each of FTC and MTC only one each of them was positive for Gal-3 immunostain. The three cases, one each of insular carcinoma, undifferentiated carcinoma and metastatic carcinoma showed positive immunostaining.
The most common benign neoplasm was FA, 17 cases (85%). Gal-3 expression in FA was absent in 16/17 cases (94%), while positivity was observed in one case (6%). With a p-value of 0.0139, that is statistically significant; the tendency of positive expression is seen to be more in FVPTC than in FA. However, no statistical correlation was established in comparing the expression of Gal-3 in FA and FC. Out of two cases of Hurthle cell adenoma, one was negative and the other was positive for Gal-3 expression. Single case of hyalinizing trabecular adenoma showed intense Gal-3 positive staining.
Discussion
Gal-3 has been found to be a promising molecular marker among the recent ancillary techniques for evaluation of thyroid neoplasms. However its exact role in thyroid tumour biology is still unknown. It has been reported that Gal-3 controls beta catenin stimulation of cyclin D1 and c-myc expression, thereby functioning as a regulator of cell cycle [12]. It is also reported to exhibit an antiapoptotic activity, thereby promoting survival of malignant cells [3,7]. Our study results support earlier reports that have shown Gal-3 over expression in malignant tumours of the thyroid [2,3]. The emergence of Gal-3 as a desirable molecular target for inhibition of apoptosis is emphasized in the literature [7].
Any study reporting on Gal-3 expression necessitates a precise and reliable histological diagnosis of the thyroid tumour [8]. When the histologic feature are equivocal, lack of consensus even among experts has led to serious concern regarding the possibility of under diagnosis of a given carcinoma or overdiagnosis of a benign neoplasm [13]. Similar to the study by Bartolazzi et al., independent evaluation of both histological and IHC specimens by two pathologists was done in our study [14]. The routine H&E stained histologic sections revealed unambiguous morphology in all our cases, yielding complete agreement of diagnosis between the two pathologists. The distinction between malignant and benign thyroid tumours is critical for further treatment and long term management of the patient. Studies have found Gal-3 to be the most sensitive and accurate marker for thyroid carcinoma [15,16]. Park et al., assessed IHC staining for six markers including Gal-3 in 295 thyroid lesions and concluded that Gal-3 is the most specific and sensitive marker of all the markers analysed [17]. Prasad et al., evaluated Gal-3 immunostaining in neoplastic (106 cases) and non-neoplastic thyroids separately [15]. The sensitivity and specificity for differentiating benign from malignant thyroid tumours reported by them was 92% and 90% as compared to 86.66% and 85% respectively in our study.
Majority of studies have reported Gal -3 positivity in 90% to 100% of papillary carcinoma cases [6,8,10,14–18]. Our study also showed that Gal -3 expression was significantly higher (91.3%) in papillary carcinoma. In few studies who have reported Gal-3 expression in PTC by histological subtype, positivity was identified in 82% to 100% cases of classic variant of papillary carcinoma [8]. Weber et al., reported 92% Gal-3 positivity in classic variant of PTC which is in concordance with the present study (93%) [5]. Gal-3 positivity in FVPTC in majority of the studies [8] is more than 75%. In the present study Gal -3 expression in FVPTC was noted in 75% of cases. No significant difference was found between FVPTC and classic PTC for the expression of Gal-3 in the study of 181 PTC’s by Cvejic et al., [6]. Gal -3 positivity in papillary micro carcinoma noted in 100% of cases in our study similar to that observed by Cvejic et al., supports the suggestion that alteration of Gal-3 expression is an early event in PTC progression and thus may be involved in tumourigenesis [6].
Expression of Gal -3 ranged from 20% to 100% in reported cases of FTC [8]. Xu XC et al., and the largest series reported by Bartolazzi et al., identified Gal-3 expression in 100% and 95% of FTC cases respectively [14,19]. Fernandez et al., and Cvejic et al., reported positivity in 50% and 64% cases of FTC respectively which was in concordance with the present study (50%); data indicating that, still there are some Gal-3 negative carcinomas [6,9]. In the present study, Gal -3 expression was noted in 50% cases of MTC which is in concordance with the studies by Fernandez and Bartolazzi who also reported 50% positivity in their cases [9,14]. Gal -3 is supposed to be not useful for detecting MTC, in keeping with their origin from a different cell line [14,19]. Studies have noted variable Gal-3 expression in poorly differentiated thyroid cancers also [4,14]. However, in majority of cases (75%to 100% of reported cases) of ATC, Gal-3 positivity was identified suggesting that differentiated thyroid carcinoma can progress or undergo anaplastic transformation into ATC [8,14]. In the present study and study by Herrmann et al., small number of cases of MTC, ATC and insular carcinoma are reported with inconsistent Gal-3 expression, making diagnostic application of Gal-3 in these rare histological subgroups unlikely [10].
Although biological studies have implicated a more prominent role for cytoplasmic localized Gal-3 in tumourigenesis and metastasis, occasional reports have compared the evaluation of Gal-3 nuclear staining and cytoplasmic staining for their ability to diagnose thyroid cancer [8]. Liu YY et al., reported that cytoplasmic Gal-3 staining identified a higher proportion of PTC cases when compared with nuclear staining [20]. Orlandi et al., concluded that cytoplasmic Gal-3 expression is evident only in malignant cells, regardless of nuclear immunostaining and hence nuclear positivity alone is not to be considered as a malignant feature [16]. In our study, predominant cytoplasmic staining was noted in all malignant neoplasms, whereas both cytoplasmic and nuclear immunostaining was seen in few cases of PTC.
Majority of the studies identified Gal-3 positive expression in between 0% and 30% of cases of follicular adenomas [8]. The present study identified Gal-3 positivity in a single case of follicular adenoma (6%). Galectin-3 positivity in the current study was also noted in a single case of Hurthle cell adenoma and hyalinizing trabecular adenoma. A study of 58 cases of Hyalinizing trabecular tumours showed Gal-3 immunostaining strong positivity in 40% and negative or weak staining in 60% of the cases [21].
The false positive Gal-3 expression in benign thyroid lesions and in normal thyroid tissue noted in variable frequencies in the literature [15] is attributed to variations in antigen retrieval protocols, antibody characteristics, antibody dilution, marker localization, and criteria for positive expression [8,17]. Researchers recommend the use of purified monoclonal antibody and a biotin free detection system [8,10]. Unlike many earlier studies [3,5,6,15,16,18,19], which have used avidin-biotin based immunoperoxidase, in our study polymeric HRP linker antibody conjugates has been used. Studies utilizing an avidin-based detection system without biotin blockade should be interpreted with caution since the thyrocytes have a high level of endogenous biotin [8,17]. Chiu et al., in their review of Gal-3 expression studies in thyroid tissue, highlight the necessity of a standardized protocol for widespread clinical application of Gal-3 expression as a diagnostic tool for differentiating malignant and benign thyroid lesions [8]. Aiad et al., assume that at least a part of the Gal-3 positive adenomas may represent an incipient malignant transformation [3].
The sensitivity, specificity, positive predictive value and negative predictive value of Gal-3 expression in thyroid neoplasms in this study was 86.67%, 85%, 89.66% and 80.95% as compared to 79%, 69%, 87%, and 56% respectively in the study by Weber KB et al., [5]. The results from our study in agreement with most of the studies, underscores the high sensitivity of Gal -3 IHC marker in the distinction of malignant and benign thyroid tumours [Table/Fig-6].
Comparative analysis of Galectin-3 protein expression in Thyroid neoplasms
| Study | PTC | FTC | MTC | Insular Carcinoma | ATC | FA |
|---|
| Weber et al., [5] | 91.7% | 44% | - | - | - | 31% |
| Prasad et al., [15] | 94% | 66% | - | - | 100% | 10% |
| Park et al., [17] | 99% | 64% | - | - | - | 2.9% |
| Xu et al., [19] | 100% | 100% | 37.5% | - | - | 0% |
| Orlandi et al., [16] | 100% | 100% | - | - | - | 10% |
| Bartolazzi et al., [14] | 97% | 95% | 43% | 65% | 90% | 3% |
| Felichenfeldt et al., [22] | 100% | 20% | - | - | - | 20% |
| Present study | 91.3% | 50% | 50% | 100% | 100% | 6% |
Limitations
However, there are a few limitations of the present study. The histomorphological study of Gal-3 expression if combined with the cytological evaluation of the cases would have provided additional evidence for the diagnostic utility of Gal-3 in thyroid cancer. Even though our study documented lower Gal-3 expression in FA compared to FTC and FVPTC, the number of cases of FTC and FVPTC are very less to arrive at a significant observation about the follicular patterned thyroid carcinomas.
Conclusion
In conclusion, Gal-3 is a good marker of malignancy especially in supporting the diagnosis of PTC and its variants. The differential expression of of Gal-3 in thyroid carcinoma compared with benign neoplasms may also represent a promising target for therapy of thyroid cancers. A reliable and reproducible Gal-3 testing methodology and positive immunoexpression criteria guidelines are necessary as it bears impact on the accurate and consistent reporting of Gal-3 expression in thyroid lesions. Thyroid carcinomas can test negative for Gal-3, hence we cannot depend on Gal-3 expression alone as a single diagnostic tool to detect thyroid malignancy.
I Score: Intensity score graded as 0 (no staining),1+ (slight staining), 2+ (moderate staining), or 3+ (intense staining)
P Score: Proportion of stained cells scored as 1+ (<5%of cells), 2+ (5%-50% of cells), or 3+ (>50% of cells)
Positive result= specific staining of more than 5% of the tumour cells with slight, moderate or intense staining