Cyclophosphamide is a potent DNA alkylating agent used in chemotherapy and immunosuppression. Although an old agent, its use in the present day has expanded for cases of refractory autoimmune disease. In this report, a case of haemorrhagic myopericarditis resulting from high-dose cyclophosphamide for chronic inflammatory demyelinating polyneuropathy is presented. The patient had no predisposing cardiovascular risk factors and a structurally normal heart on previous echocardiogram. Following administration of high-dose cyclophosphamide, the patient developed acute congestive heart failure. Serial echocardiography demonstrated pericardial effusion, myocardial thickening, and progressive right ventricular dysfunction. Histopathology on autopsy revealed acute myocardial necrosis, intra-myocardial extravasation of blood, fibrin, and fibrin-platelet microthrombi compatible with the diagnosis of haemorrhagic myopericarditis. The ante-mortem diagnostic dilemma is described to emphasize the need for pattern recognition and clinical criteria for diagnosis. Subsequent comprehensive literature review was performed to identify features that will facilitate earlier diagnosis of haemorrhagic myopericarditis by healthcare providers.
Case Report
A 41-year-old woman presented with dyspnea after receiving cyclophophamide (CYA) 50 mg/kg, for four day, for treatment of refractory Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). Prior to initiation of therapy, she had no history of coronary artery disease or congestive heart failure, and had a structurally normal heart by echocardiography 6 months previously. She had never been placed on prior chemotherapy for her disease (only trials of intravenous immunoglobulin and steroids) and had no other predisposing cardiovascular risk factors. On day 4 of CYA therapy, she developed dyspnea and lower extremity oedema with troponin elevation to 2.08 ng/mL (peak 7.51 ng/mL) and new diffuses low voltage waves on electrocardiogram. Chest radiograph showed progressive pulmonary vascular distention with cardiomegaly. Echocardiogram evidenced diffuse myocardial thickening and moderately sized circumferential pericardial effusion with reduced right ventricular expansion and early right atrial collapse, concerning for early tamponade physiology [Table/Fig-1].
Resting EKG and chest radiograph prior to CYA: a) and on day 4 after first dose, b) Echocardiogram with new myocardial thickening and pericardial effusion on day 5, c) Although EKG and chest radiography show left atrial enlargement and poor R wave progression, prior echocardiogram demonstrated structurally normal heart. Left ventricular thickening was new compared to prior echocardiogram
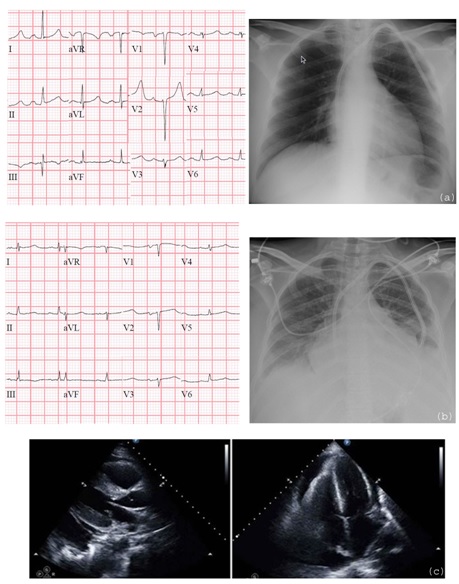
She required pericardiocentesis on day 10 for increasing effusion size. Despite effusion drainage, Right Ventricular (RV) systolic function deteriorated on serial echocardiograms although her left ventricular function remained intact. She progressed to multi-organ failure including liver and kidney. Her diagnosis remained in question as competing diagnoses included acute coronary syndrome versus progression of rheumatologic disease. A diagnosis of CYA haemorrhagic myopericarditis could not be established by MRI (due to renal failure) or by biopsy (due to progressive pancytopenia and multi-organ failure). A diagnosis of haemorrhagic myopericarditis was questioned due to focal right ventricular dysfunction with preservation of left ventricle ejection fraction.
24 days after day 1 of CYA therapy, she progressed to Pulseless Electrical Activity (PEA) arrest and died. Autopsy revealed cardiomegaly (530 g), acute myocardial necrosis, intra-myocardial extravasation of blood, fibrin, and fibrin-platelet microthrombi within capillaries. The histopathologic findings were compatible with a diagnosis of haemorrhagic myopericarditis and were most prominent in the myocardium of her right ventricle, consistent with the progressive structural RV changes seen on echocardiography.
Discussion
Reports of CYA-cardiotoxicity date back to case series from as early as 1976 describing high mortality with acute haemorrhagic myopericarditis [1,2]. Despite its history, there is no consensus for diagnosis or treatment for haemorrhagic myopericarditis. The drug is still being used in combination chemotherapy as well as expanded application for autoimmune conditions, such as CIDP in our patient. The potentially devastating effects of this drug demand attention, particularly by physicians who might be less experienced with its use.
Our case was complicated by difficulty in establishing an ante-mortem clinical diagnosis. Many consulting physicians were involved in this patient’s care and the competing diagnoses delayed treatment initiation. Our patient never received a trial of therapy for haemorrhagic myopericarditis. Early diagnosis of haemorrhagic myopericarditis on clinical presentation is critical both for treatment and prognosis, especially given the rapidly fatal course. Since most literature pre-dates use of Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) and biopsy is not technically feasible under profound myelosuppression or multi-organ dysfunction, clinicians need a diagnostic criteria for haemorrhagic myopericarditis.
In contrast to previous case series depicting worsening left ventricular function with high-dose cyclophosphamide [3,4], our patient had the atypical presentation of structural changes primarily involving the right ventricle with overall preserved LV function. These findings were supported by histopathology revealing greater myocardial damage within the RV. The explanation for the uneven insult favoring the RV in our patient remains unclear. No predisposing factors have been identified to support involvement of specific chambers following high-dose cyclophosphamide.
To identify common clinical patterns of haemorrhagic myopericarditis, reports of cyclophosphamide cardiotoxicity were searched using PubMed/MEDLINE for keywords “cyclophosphamide,” “cardiotoxicity,” “haemorrhagic myopericarditis.” Fourteen articles [1–14] from 1976 to 2014 met literature search criteria [Table/Fig-2].
Literature review of haemorrhagic myopericarditis cases
| Article | Year | Article Type | Total Patients | “High Dose” of CYA | Hemorrhagic Myopericarditis Cases | CHF onset | Troponin Elevation | Patients with Loss of Voltage on EKG | Patients with Pericardial Effusion |
|---|
| Appelbaum et al., [1] | 1976 | Case Series | 15 | 45 mg/kg over 4 days | 4 | 5-9 days | - | 4 | 4 |
| Mills et al., [2] | 1979 | Case Series | 2 | 36-42 mg/kg over 4 days | 2 | 4 days | - | 2 | 2 |
| Gottdiener et al., [3] | 1981 | Prospective | 32 | 45 mg/kg over 4 days | 9 | <21 days | - | 29 | 5 |
| Goldberg et al., [4] | 1986 | Retrospective | 84 | 50 mg/kg over 4 days | 14 | <10 days | - | - | - |
| Kupari et al., [5] | 1990 | Prospective | 45 | 60 mg/kg over 2 days | 2 | 10 days | - | 12 | 4 |
| Braverman et al., [6] | 1991 | Prospective | 44 | 1.8 g/m2/d 1.5 g/m2/d 1.5 g/m2/d over 4 days | 4 | 7-17 days | - | 16 | - |
| Lee et al., [7] | 1996 | Case Series | 13 | 60 mg/kg over 2 days 80 mg/kg over 2 days 50 mg/kg over 4 days | 13 | 4-9 days | - | 9 | 9 |
| Nakamae et al., [8] | 2000 | Prospective | 19 | 1.4 g/m2/d over 4 days | 5 | 5-12 days | - | - | - |
| Birchall et al., [9] | 2000 | Case Report | 1 | 1.6 g/m2/d over 4 days | 1 | 11 days | - | - | 1 |
| Morandi et al., [10] | 2001 | Prospective | 16 | 163.5 mg/kg over 1 day | 0 | - | 0 | 6 | 1 |
| Auner et al., [11] | 2002 | Prospective | 30 | 60 mg/kg over 2 days | 0 | - | 0 | 11 | - |
| Kamezaki et al., [12] | 2005 | Case Report | 1 | 50 mg/kg over 2 days | 1 | 3 days | + | 1 | 0 |
| Zver et al., [13] | 2007 | Prospective | 23 | 4 g/m2/d over 1 day | 0 | - | 0 | - | - |
| Katayama et al., [14] | 2009 | Case Report | 1 | 1.5 g/m2/d over 2 days | 1 | 6 days | + | 1 | 1 |
Dose: The cases listed in [Table/Fig-2] defined “high-dose” cyclophosphamide as 40 mg/kg/d or 1.5 g/m2/d for 4 days, although 1 case [2] occurred at a dose of 36 mg/kg for 4 days and 16 patient cases after only 2 days of CYA [5,7,12,14]. Furthermore, Nakamae et al., reported a 26% incidence of CHF with only 1.4 g/m2/d over 4 days [8]. Based on the studies, “high-dose” leading to a higher predisposition of developing haemorrhagic myopericarditis could be defined as 40 mg/kg/d or 1.4 mg/kg/d over at least 2 days. It is important to note that toxicity does not occur at lower doses which are usually administered in patients with inflammatory diseases or vasculitis [Table/Fig-3].
Proposed clinical criteria for early diagnosis of cyclophosphamide haemorrhagic myopericarditis
| MUST BE PRESENT: |
|---|
| 1. | Dosage: greater than 40 mg/kg/d OR 1.4 g/m2/d over minimum 2 consecutive days |
| 2. | CHF symptom onset: 3-12 days after first dose |
| 3. | Troponin I elevation in the absence of angina: minimum peak greater than 0.75 ng/mL |
| 4. | EKG Changes: new diffuse low voltage (amplitude of entire QRS complex {R+S} <10 mm in all precordial leads and <5 mm in all limb leads) |
| ONE TYPE OF IMAGING OR DIAGNOSTIC MODALITY |
| - | Echocardiography: new pericardial effusion, increased intraventricular septal thickening during diastole, change in E/A ratio (diastolic dysfunction), early functional mitral regurgitation (Note: Changes in LVEF may be delayed in clinical course or isolated to one ventricle and thus should not be used in early diagnosis of CYA haemorrhagic myopericarditis) |
| - | Cardiac MRI |
| - | Biopsy: intramyocardial extravasation of blood, fibrin, or fibrin-platelet microthrombi in capillaries, and fibrin strands in interstitium |
Clinical Presentation: Clinical presentation is the hallmark of for early recognition of CYA-cardiotoxicity. Haemmorrhagic myopericarditis will present with typical CHF symptoms, including new dyspnea at rest, elevated jugular venous pulsations, atypical chest pain, and peripheral oedema. Chest radiograph often supports findings of fluid overload. In the summarized studies, these symptoms most often occurred soon after completion of a 4-day high-dose regimen, almost always within 11 days.
Cardiac Enzymes: Evaluation of cardiac enzymes were not described in earlier series as they pre-dated routine use of sensitive or specific biomarkers such as cardiac troponin I (CnI). Two studies [10,11] evaluated cardiac troponin following CYA. No cases of haemorrhagic myopericarditis were identified in these studies and troponin levels were never elevated, suggesting adequate negative predictive value. In contrast, Cardinale et al., identified CnI levels of 0.75 ng/mL were associated with cardiac damage from combination chemotherapy regimens [15]. Although Brain Natriuretic Peptide (BNP) was studied in two reports [8,9], elevations in patients with acute heart failure were identified 14 days after clinical signs had already developed. Thus BNP is not an ideal sensitive marker for early diagnosis. Finally, BNP implies stretch of the myocardium, whereas troponin elevations imply intrinsic myocyte damage, the latter of which is the fundamental process of haemorrhagic myopericarditis from cyclophosphamide.
Electrocardiography: EKG changes were often reported as summated QRS values, although two articles [8,11] identified QT dispersion as an independent predictor for the development of heart failure prior to echocardiographic findings. Early voltage loss occurred in approximately 90% of haemorrhagic myopericarditis cases as early as 1-3 days and peaking around 7 days post-treatment. In one study, voltage loss preceded LV dysfunction but returned to baseline by 21 days if the patient survived [3]. The findings of voltage loss are not specific, given that Kupari et al., found at least 15% reductions in summated QRS from pre-treatment EKG in 27% of patients receiving 60 mg/kg for 2 days with a smaller proportion developing clinical heart failure [5].
Imaging: In the diagnostic criteria proposed [Table/Fig-3], at least one radiographic or histopathologic investigation should also be employed. Unfortunately, echocardiography is not reliable to identify early effects of CYA since reductions in Left Ventricular Ejection Fraction (LVEF) can often be delayed and pericardial effusion is not specific. Additional echocardiographic findings that have previously been associated with haemorrhagic myocarditis in the right clinical setting are reported in [Table/Fig-3] [10,12]. MRI is established as a useful modality for myocarditis [16]. However, the delay in progression of LV dysfunction and the paucity of data for expected MRI findings in this specific scenario limits the yield. Moreover, MRI may not be feasible in unstable patients with co-morbid renal disease.
Histopathology: Tissue pathology demonstrating specific findings of intra-myocardial extravasation of blood, fibrin, fibrin-platelet microthrombi in capillaries, and fibrin strands in the interstitium [17] is the most specific finding for diagnosis of haemorrhagic myopericarditis. This much more invasive approach unfortunately has limited feasibility in many presenting patients, especially in those who are pancytopenic and coagulopathic from simultaneous bone marrow suppression (in addition to those with aforementioned co-morbid renal failure when contrast cannot be used).
At the present time, CYA haemorrhagic myopericarditis remains highly fatal and there are no standard treatment options proven to reduce mortality. Use of corticosteroids, theophylline, nonselective adenosine antagonist, ascorbic acid, or mechanical circulatory support [7,12,18,19] have been anecdotally reported and must be guided by clinical judgment. No reports were found to demonstrate protection with use of co-administration of mesna. The case and criteria presented here will provide physicians with the tools for earlier diagnosis with potential prognostic implications when treating patients on high-dose cyclophosphamide.
Conclusion
Cyclophosphamide is a potent agent with expanded application in the present day. Use of high-dose cyclophosphamide can be associated with lethal haemorrhagic myopericarditis, although is unlikely to have toxic effects at more commonly used doses for inflammatory conditions and vasculitis. The patient in this report demonstrated potential devastating effects from high-dose therapy, which needs to be recognized as potential risk of therapy. Early recognition and diagnosis of haemorrhagic myopericarditis is necessary to reduce mobidity and morbidity associated with toxicity. By appreciating patterns previously reported in the literature, the diagnostic criteria presented here will guide physicians to facilitate an early clinical diagnosis.
[1]. Appelbaum FR, Strauchen JA, Graw RG, Savage DD, Kent KM, Ferrans VJ, Acute lethal carditis caused by high-dose combination chemotherapy: a unique clinical and pathological entityLancet 1976 1:58-62. [Google Scholar]
[2]. Mills BA, Roberts RW, Cyclophosphamide-induced cardiomyopathy: a report of two cases and review of the English literatureCancer 1979 43:2223-26. [Google Scholar]
[3]. Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J, Cardiotoxicity associated with high-dose cyclophosphamide therapyArch Intern Med 1981 141:758-63. [Google Scholar]
[4]. Goldberg MA, Antin JH, Guinan EC, Rappeport JM, Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factorBlood 1986 68:1114-18. [Google Scholar]
[5]. Kupari M, Volin L, Suokas A, Timonen T, Hekali P, Ruutu T, Cardiac involvement in bone marrow transplantation: electrocardiographic changes, arrhythmias, heart failure and autopsy findingsBone Marrow Transplant 1990 5:91-98. [Google Scholar]
[6]. Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT, Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimensJ Clin Oncol 1991 9:1215-23. [Google Scholar]
[7]. Lee CK, Harman GS, Hohl RJ, Gingrich RD, Fatal cyclophosphamide cardiomyopathy: its clinical course and treatmentBone Marrow Transplant 1996 18:573-77. [Google Scholar]
[8]. Nakamae H, Tsumura K, Hino M, Hayashi T, Tatsumi N, QT dispersion as a predictor of acute heart failure after high-dose cyclophosphamideLancet 2000 355:805-06. [Google Scholar]
[9]. Birchall IW, Lalani Z, Venner P, Hugh J, Fatal haemorrhagic myocarditis secondary to cyclophosphamide therapyBr J Radiol 2000 73:1112-14. [Google Scholar]
[10]. Morandi P, Ruffini PA, Benvenuto GM, La Vecchia L, Mezzena G, Raimondi R, Serum cardiac troponin I levels and ECG/Echo monitoring in breast cancer patients undergoing high-dose (7 g/m(2)) cyclophosphamideBone Marrow Transplant 2001 28:277-82. [Google Scholar]
[11]. Auner HW, Tinchon C, Brezinschek RI, Eibl M, Sormann S, Maizen C, Monitoring cardiac function by serum cardiac troponin T levels, ventricular repolarisation indices, and echocardiography after conditioning with fractionated total body irradiation and high-dose cyclophosphamideEur J Haemtol 2002 69:1-6. [Google Scholar]
[12]. Kamezaki K, Fukada T, Makino S, Harada M, Cyclophosphamide-induced cardiomyopathy in a patient with seminoma and a history of mediastinal irradiationIntern Med 2005 44:120-23. [Google Scholar]
[13]. Zver S, Zadnik V, Bunc M, Rogel P, Cernelc P, Kozelj M, Cardiac toxicity of high-dose cyclophosphamide in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantationInt J Hematol 2007 85:408-14. [Google Scholar]
[14]. Katayama M, Imai Y, Hashimoto H, Kurata M, Nagai K, Tamita K, Fulminant fatal cardiotoxicity following cyclophosphamide therapyJ Cardiol 2009 54:330-34. [Google Scholar]
[15]. Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapyJ Am Coll Cardiol 2000 36:517-22. [Google Scholar]
[16]. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, Cardiovascular magnetic resonance in myocarditis: a JACC white paperJ Am Coll Cardiol 2009 53:1475-87. [Google Scholar]
[17]. Buja LM, Ferrans VJ, Graw RJ, Cardiac pathologic findings in patients treated with bone marrow transplantationHum Pathol 1976 7:17-45. [Google Scholar]
[18]. Singal PK, Iliskovic N, Doxorubicin-induced cardiomyopathyN Engl J Med 1998 339:900-05. [Google Scholar]
[19]. Freilich M, Stub D, Esmore D, Negri J, Salamonsen R, Bergin P, Recovery from anthracycline cardiomyopathy after long-term support with a continuous flow left ventricular assist deviceJ Heart Lung Transplant 2009 28:101-03. [Google Scholar]